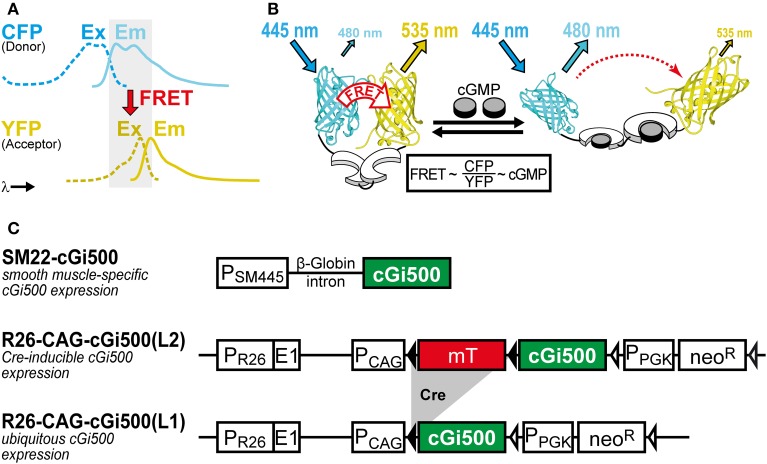
Figure 1.
(A,B) Working principle of the FRET-based cGi500 biosensor and (C) transgenes used to generate cGi500-expressing mice. (A) Spectral overlap (gray) of YFP excitation (Ex, dashed lines) and CFP emission (Em, solid lines) spectra that is necessary for FRET to occur. (B) The cGMP indicator protein cGi500 consists of the tandem cGMP-binding sites from bovine cGK type I (white) flanked by CFP and YFP. Without cGMP, FRET occurs from excited CFP to YFP, leading to light emission from YFP. Binding of cGMP (gray) causes a conformational change and a decrease in FRET efficiency, so that light emission from YFP at 535 nm is reduced and emission from CFP at 480 nm is increased. (B) is reproduced from Thunemann et al. (2013b). (C) Constructs used to generate transgenic cGi500-expressing mice. Abbreviations: E1, first exon of the endogenous Rosa26 gene; mT, membrane-targeted tandem-dimer tomato red fluorescent protein; neoR, neomycin resistance gene; PCAG, chicken actin/β-globin promoter; PPGK, phosphoglycerate kinase promoter; PSM445, 445-bp promoter fragment of the Transgelin/SM22 gene; PR26, endogenous Rosa26 promoter. Black triangles represent loxP sites, open triangles represent FRT sites. See also Section “Selection of Appropriate Biosensors and Generation of Biosensor-Expressing Mice” for further details on mouse generation and characterization.