Fig. 3. Structural analysis of post-translationally modified SOS proteins reveals catalytically competent and non-competent protein conformations.
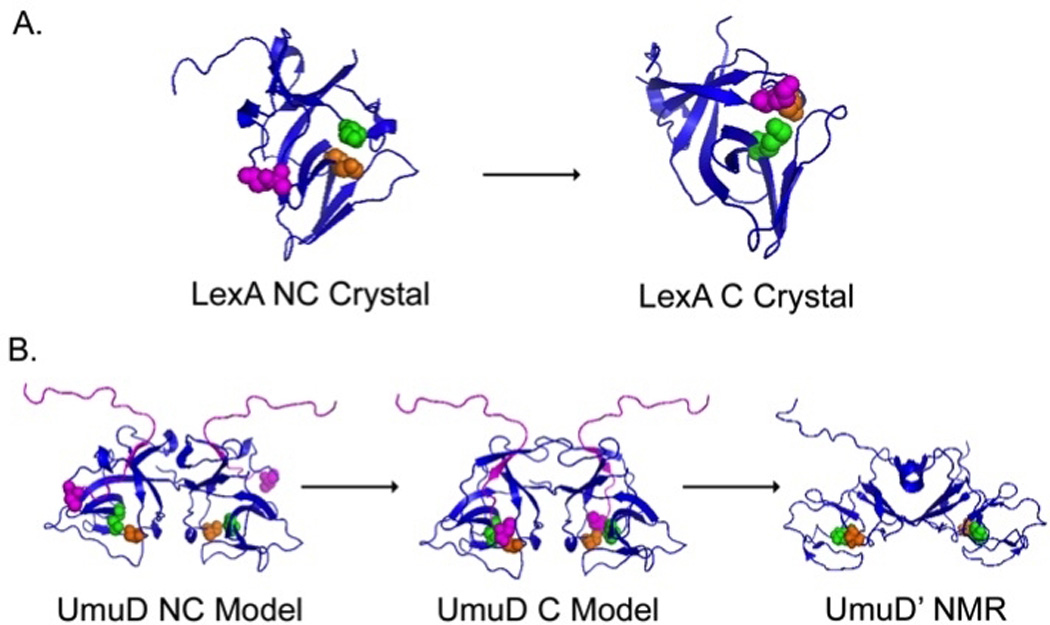
LexA (A) and UmuD (B) proteins undergo a large rearrangement from a non-cleavable conformation (NC) to a cleavable conformation (C). A. LexA crystal structures indicate that the Ala84-Gly85 residues (purple) can be positioned 20 Å away from Lys156 (green) and Ser119 (orange) in the NC form (left) to a position allowing for an autoproteolytic cleavage event in the cleavable form (right) (227). B. Full length models of the non-cleavable (left) and cleavable (middle) UmuD2 dimer. The N-terminal arms of UmuD2 (purple) fold to present the cleavage site (C24 purple spheres) to Ser60 (orange) and Lys97 (blue) (compare left and middle). NMR data suggest that after the cleavage event forming UmuD’2 (right), the dimer undergoes a significant conformational change that consequently alters cellular activity (112, 284, 365).
