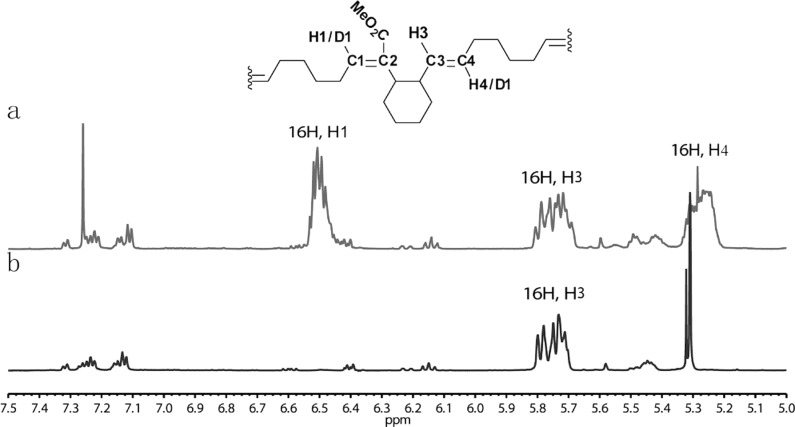
Figure 3.
Alternating ring-opening metathesis polymerization (AROMP) of monomers 4 and 6. The region of 1H NMR spectra in which backbone olefinic hydrogen resonances of poly(4-alt-6)n appear is shown. (a) Polymer product prepared from cyclohexene and dissolved in CDCl3. The ratio of H1:H3:H4 is 1:1:1. (b) Polymer product prepared from cyclohexene-d10 and dissolved in CD2Cl2. The ratio of H1:H3:H4 is 0:1:0. poly(4-alt-6-d10)n was dissolved in CD2Cl2 instead of CDCl3 to allow accurate integration of the phenyl resonances.