FIGURE 1:
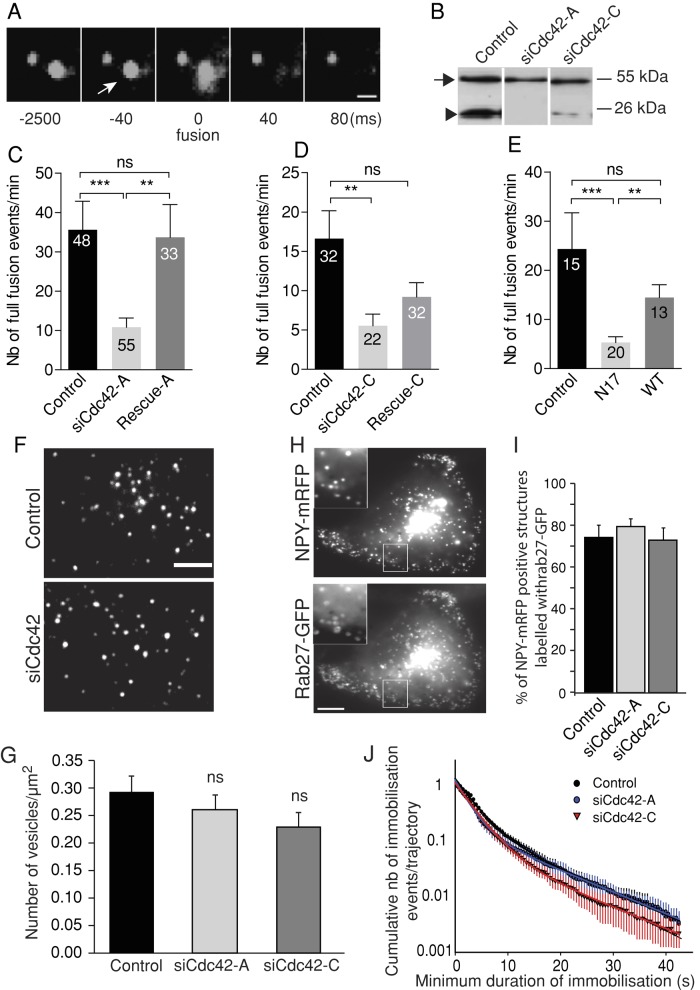
Cdc42 knockdown impairs full fusion but has little effect on SG recruitment at the plasma membrane. BON cells were transfected with a vector encoding NPY tagged with a fluorescent protein (mRFP, GFP, or pHluorin, a pH-sensitive GFP variant) and with control or siCdc42 siRNA duplexes. (A) A typical full-collapse exocytotic event captured by TIRFM. The fluorescence of the vesicular content marker NPY-GFP briefly increases upon exocytosis (arrow) and then decays to background levels as NPY-GFP diffuses. Bar, 1 μm. Time is indicated in milliseconds. In this example, the acquisition rate was >10 Hz, as used elsewhere in this study. (B) A representative Western blot showing reduced Cdc42 levels (arrowhead) in siCdc42-A– and siCdc42-C–treated BON cells. The observed reduction was 90.1 ± 2.7% (p < 0.001) with siCdc42-A (n = 8 independent experiments) and 68.9 ± 12.7% (p < 0.001) with siCdc42-C (n = 10 independent experiments). Tubulin staining (arrow) was used to normalize the Cdc42 signal. (C, D) Cdc42 knockdown decreases the number of full-fusion events. The mean (±SEM) number of events observed per cell in the different conditions: cells treated with control (luciferase-targeting siRNAs), siCdc42-A (C), or siCdc42-C (D) siRNAs. Coexpressing HA-tagged Cdc42 constructs insensitive to siCdc42-A (rescue-A) or to siCdc42-C (rescue-C) restored at least partially the secretory responses. For imaging, cells were selected on the basis of their NPY-mRFP fluorescence. Rescue-A and rescue-C were not visible in live cells, but control experiments revealed that they were consistently expressed in cells cotransfected with NPY-mRFP. The number of analyzed cells is indicated in the bars. **p < 0.01; ***p < 0.001 (Kruskal–Wallis followed by Dunn's test); ns, non significant. (E) Overexpression of mCherry-tagged Cdc42-N17, but not mCherry-Cdc42 WT, decreases the occurrence of full-fusion events compared with control cells expressing only mCherry. (F, G) The spatial distribution of SGs was analyzed by TIRFM under resting conditions in control or siCdc42-treated cells. (F) Representative images. (G) The juxtamembrane SG density was not significantly affected by Cdc42 knockdown (n = 18 cells from three independent experiments in each group). The size of cell footprints was not modified by Cdc42 silencing. (H, I) Confocal imaging of BON cells revealed that the majority of NPY-mRFP–positive structures are also labeled by GFP-Rab27a, which is associated with the cytoplasmic side of the SG membrane. This proportion was not affected by Cdc42 knockdown, as indicated by the quantification shown in I (mean ± SEM; n = 12 cells in each condition), suggesting unimpaired SG biogenesis. Representative images of SiCdc42-C-treated cells. Insets are zoomed-in views of the boxed areas. Bar, 5 μm. (J) Single SGs imaged by TIRFM were tracked, and the Dxy values were computed along trajectories using a rolling analysis window. Subtrajectories characterized by a Dxy lower than the threshold value of 5 × 10−4 μm2/s were defined as an immobilization period. A survival plot of immobilization events is shown. Control, 19 cells, 2978 trajectories; siCdc42-A, 18 cells, 2283 trajectories; siCdc42-C, 17 cells, 2917 trajectories; from three independent experiments. Data were fitted with the sum of two exponentials. Cdc42 silencing did not significantly alter SG immobilization, suggesting unimpaired SG attachment at the plasma membrane.