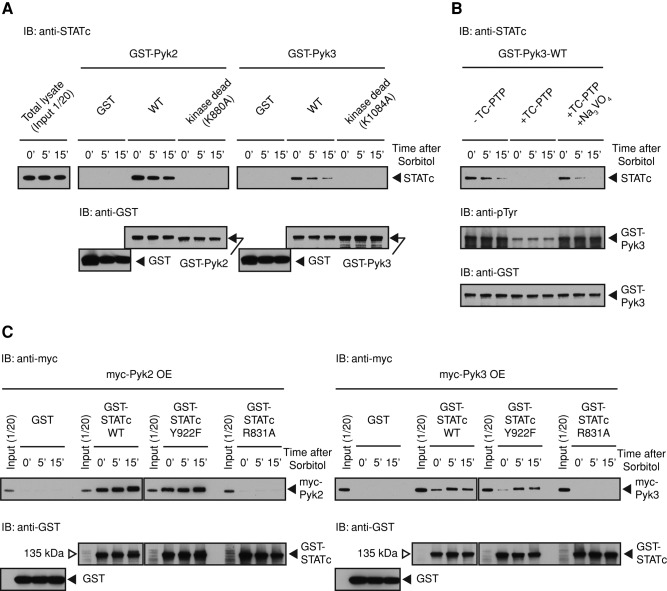
FIGURE 6:
Pyk3 and STATc physically interact. (A) Analysis of the interaction of GST-Pyk2 and GST-Pyk3 with STATc. Ax2 cells were developed in suspension for 4 h and then left untreated or exposed to sorbitol as in Figure 2A. Cells were lysed, and the extracts were subjected to pull-down assay using GST-Pyk2-WT or GST-Pyk2-K880A, its kinase-dead form, and GST-Pyk3-WT or GST-Pyk3-K1084A. Bound STATc was detected using total STATc antibody 7H3. The blot was reprobed with a GST antibody as a loading control. (B) Tyrosine phosphorylation–dependent GST-Pyk3 binding to STATc. GST-Pyk3 was bound to glutathione beads, and the beads were either left untreated or digested with TC-PTP. A parallel reaction was performed on GST-Pyk3 beads in the presence of sodium orthovanadate, an inhibitor of the enzyme, left untreated or induced with sorbitol, and assayed as in A. The blot was reprobed with a GST antibody as a loading control, and the same samples were reanalyzed with a general phosphotyrosine antibody 4G10. (C) Pull-down analysis of Pyk3 by parental and mutant forms of STATc. Myc-Pyk2 OE or myc-Pyk3 OE cell lysate from control cells, or cells induced with sorbitol, were subjected to pull-down assay using GST-STATc or its R831A and Y922F mutant forms as in A. The blot was reprobed with a GST antibody as a loading control.