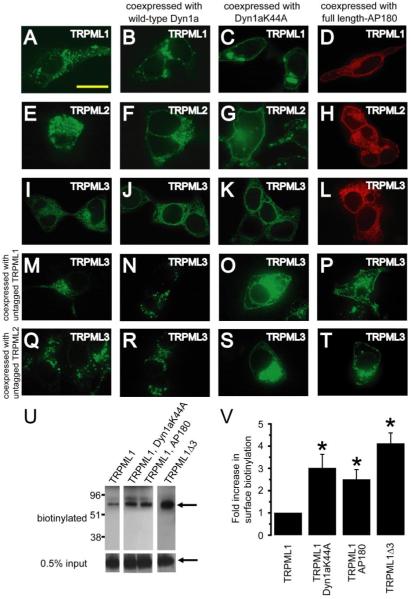
FIGURE 7. Coexpression with mislocalized TRPML1 or TRPML2 causes mislocalization of TRPML3.
A, confocal image of fixed HEK293 cells that were previously transfected with TRPML1-YFP at the excitation wavelength of 488 nm. B and C, same as A but in HEK293 cells co-transfected with: TRPML1-YFP and Dyn1aWT (B) or Dyn1aK44A (C). D, confocal image of HEK293 cells co-transfected with TRPML1-HA and full-length AP180 and in which immunofluorescence was performed with anti-HA primary antibodies and Alexa568-conjugated secondary antibodies and viewed at an excitation wavelength of 568 nm. E–H, same as A–D but with TRPML2-YFP instead of TRPML1-YFP. I–L, same as A–D but with TRPML3-YFP instead of TRPMl1-YFP. M–P, confocal images of HEK293 cells co-transfected with vectors encoding the following and viewed at an excitation wavelength of 488 nm: TRPML3-YFP and TRPML1 (M); TRPML3-YFP, TRPML1 and wild-type Dyn1a (N); TRPML3-YFP, TRPML1, and Dyn1aK44A (O); and TRPML3-YFP, TRPML1, and full-length AP180 (P). Q–T, same as M–P but with TRPML2 instead of TRPML1. The scale bar represents 20 μm. U, increased biotinylation of TRPML1 due to inhibition of clathrin-mediated endocytosis and deletion of lysosomal targeting motif. The Western blot shows extracts prepared from HEK293 cells expressing TRPML1-HA alone (left lane), TRPML1-HA with Dyn1aK44A or AP180 (middle lanes), and TRPML1Δ3-HA alone (right lane). The extracts were prepared following surface biotinylation and the biotinylated proteins were purified. The blot was probed with anti-HA primary antibodies and horseradish peroxidase-conjugated secondary antibodies. Upper panels show surface-biotinylated fractions. The arrow indicates the 65-kDa TRPML1 band. The lower panels show the 0.5% input, with the arrow indicating the TRPML1 band. V, bar graphs showing the fold-increase in the TRPML1-HA surface biotinylation upon inhibition of clathrin-mediated endocytosis or as a result of mutating the lysosomal targeting motif. Averages are based on three separate experiments in each case and the error bars indicate S.E. Unpaired Student’s t tests indicate that the fold increase in surface biotinylation of TRPML1-HA was statistically significant (p < 0.05) upon coexpression with Dyn1aK44A or full-length AP180 or upon removal of the lysosomal targeting motif.