ABSTRACT
Channels are integral membrane proteins that form a pore, allowing the passive movement of ions or molecules across a membrane (along a gradient), either between compartments within a cell, between intracellular and extracellular environments or between adjacent cells. The ability of cells to communicate with one another and with their environment is a crucial part of the normal physiology of a tissue that allows it to carry out its function. Cell communication is particularly important during keratinocyte differentiation and formation of the skin barrier. Keratinocytes in the skin epidermis undergo a programme of apoptosis-driven terminal differentiation, whereby proliferating keratinocytes in the basal (deepest) layer of the epidermis stop proliferating, exit the basal layer and move up through the spinous and granular layers of the epidermis to form the stratum corneum, the external barrier. Genes encoding different families of channel proteins have been found to harbour mutations linked to a variety of rare inherited monogenic skin diseases. In this Commentary, we discuss how human genetic findings in aquaporin (AQP) and transient receptor potential (TRP) channels reveal different mechanisms by which these channel proteins function to ensure the proper formation and maintenance of the skin barrier.
KEY WORDS: Aquaporin, Gap junction, TRP channel
Introduction
The skin barrier
There are a number of components of the skin barrier (Proksch et al., 2008). The physical barrier of the skin is formed mainly by the stratum corneum, which consists of dead keratinocytes, or corneocytes, that are embedded in an extracellular matrix formed of lipid bilayers (Fig. 1). In corneocytes, the cell membrane is replaced by a tough protein and lipid envelope (the cornified cell envelope) that provides mechanical strength to the stratum corneum, whereas the lipid matrix forms the main permeability barrier against the invasion of bacteria and other hazardous substances. However, the living nucleated keratinocytes of the lower granular and spinous layers (the stratum granulosum and stratum spinosum, respectively) also provide an important component to the skin barrier, through the formation of cell–cell adhesion junctions, including desmosomal junctions, tight junctions and adherens junctions (Fig. 1B), which link to the cell cytoskeleton, thereby providing additional strength and restricting paracellular permeability, including limiting water loss from the body.
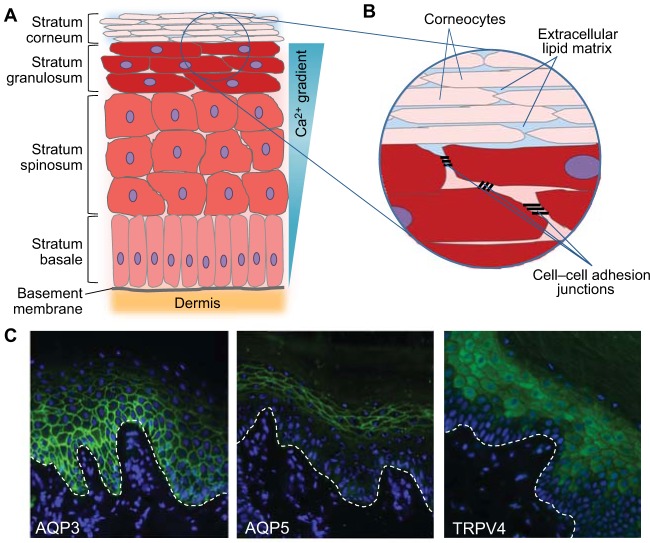
Fig. 1.
Structure of the epidermis and epidermal barrier and localisation of AQP3, AQP5 and TRPV4 in normal palmar skin. (A) Illustration of the different layers of keratinocytes found in the epidermis. Keratinocytes in the stratum basale (the deepest layer of the epidermis) are attached to the basement membrane and undergo proliferation. Upon leaving the stratum basale, keratinocytes stop proliferating and enter a programme of terminal differentiation. These cells move up through the stratum spinosum and stratum granulosum layers of the epidermis, undergoing a conformational change as they do so. In the outer layer of the epidermis, the stratum corneum, the keratinocytes are flattened anucleated dead cells known as corneocytes, which are continuously lost from the surface of the skin and replenished by cells from the lower layers of the epidermis. Ca2+ is essential for the regulation of keratinocyte differentiation, and a Ca2+ gradient is seen in the epidermis. (B) The epidermal barrier is formed from a number of components. The stratum corneum forms the main physical barrier of the skin and consists of corneocytes, with a toughened cornified cell envelope, embedded in an extracellular lipid matrix, which forms the main permeability barrier. Cell–cell adhesion junctions, including desmosomal junctions, tight junctions and adherens junctions, between the living nucleated keratinocytes of the lower granular and spinous layers also provide strength to the skin barrier and restrict paracellular permeability. (C) Localisation of some key aquaporin and TRP channel proteins in normal palmar skin is shown using immunofluorescent staining. AQP3 (green, left panel) is expressed in the basal and spinous layers of the epidermis, but AQP3 expression is lost in the upper granular layer, consistent with a role for AQP3 in keratinocyte proliferation and negative regulation of differentiation. By contrast, AQP5 (green, middle panel) shows a strong plasma membrane localisation in keratinocytes of the granular layer, indicating a role for AQP5 in the formation or maintenance of the epidermal barrier, although further work is required to establish the function of AQP5 in the epidermis. Similarly, TRPV4 (green, right panel) is also expressed in the upper layers of the epidermis, where it is has been shown to play a role in the formation of the intercellular junctions in the epidermal barrier. An association between TRPV4 and AQP5 has been demonstrated in other cell types, and they might have a similar relationship in keratinocytes, although further studies are required to establish this. Nuclei are shown in blue (DAPI), and the white dashed line indicates the location of the basement membrane.
The importance of gap junctions
The importance of channels in human skin became evident a number of years ago, with the identification of mutations in genes encoding various connexins, the protein subunits of gap junctions, as the underlying cause of a spectrum of inherited skin disorders that exhibit palmoplantar keratoderma (PPK) as a common feature (Scott and Kelsell, 2011). Gap junctions are major channels that allow cells to communicate with one another by facilitating intercellular communication, including the transfer of Ca2+, a fundamental ion in keratinocyte differentiation, and small molecules of <1 kDa – such as inositol triphosphate (IP3) – between cells. In the skin, gap junctions appear to regulate a number of cellular processes, including wound healing, differentiation and barrier function (Goliger and Paul, 1995; Coutinho et al., 2003; Qiu et al., 2003; Djalilian et al., 2006; Mori et al., 2006; Man et al., 2007; Kandyba et al., 2008; Simpson et al., 2013), and their role in epidermal integrity is reviewed elsewhere (Martin et al., 2014).
Connexins are four-transmembrane-domain proteins that form the subunits of gap junctions. Six connexins oligomerise in the endoplasmic reticulum (ER) or Golgi apparatus to form a connexon or ‘hemichannel’ prior to arriving at the plasma membrane or cell surface. These hemichannels can then ‘dock’ with a hemichannel on an adjacent cell to connect the cytoplasms of the respective cells, forming a gap junction. Thousands of these channels come together to form large clusters or plaques that are visible by electron microscopy (Caspar et al., 1977). Gap junctions can have differing permeabilities to molecules and ions depending on their specific connexin composition. Furthermore, hemichannels can be homomeric (made up of the same six connexins) or heteromeric (made up of two or more types of connexin), and different hemichannel combinations can dock with one another and form homotypic (two identical hemichannels) or heterotypic (different hemichannels) channels (Ahmad et al., 1999; Martin et al., 2001), adding another level of complexity to these junctions. Thus, in the epidermis, which is known to express at least 9 of the 21 known human connexins, including Cx26, Cx30, Cx30.3, Cx31 and Cx43, multiple hemichannels and gap junction types will exist (Wiszniewski et al., 2000; Di et al., 2001). This might indicate some redundancy between the connexins for their normal function in the skin, which is supported by the association of loss-of-function mutations of GJB2 and GJB6 (encoding Cx26 and Cx30, respectively) with non-syndromic autosomal recessive sensorineural hearing loss without a dermatological condition (Scott and Kelsell, 2011). However, carriers of the common recessive deafness-associated GJB2 mutations 35delG and R143W have been reported to exhibit a normal but slightly thicker epidermis (D'Adamo et al., 2009; Guastalla et al., 2009). In vitro studies, using three-dimensional (3D) skin culture models, support these histological and ultrasound observations, and suggest that loss of functional Cx26 might confer improved barrier function in the skin (and also in the gut), with increased protection from bacterial infection (Man et al., 2007; Simpson et al., 2013).
In contrast to the recessively inherited loss-of-function deafness-associated mutations, connexin mutations that cause hyperkeratotic skin disease (with or without deafness), such as Vohwinkel's syndrome (mutilating keratoderma with hearing loss), keratitis-ichthyosis-hearing loss (KID) syndrome and erythrokeratoderma variabilis (EKV), are dominantly inherited. These skin-disease-associated connexin mutants appear to either suppress wild-type gap junction channel activity by exerting a dominant-negative effect on wild-type connexins or a trans-dominant effect on other connexin proteins (Rouan et al., 2001), or represent gain-of-function variants (Richard et al., 1998; Bakirtzis et al., 2003; Essenfelder et al., 2004; Gerido et al., 2007; Lee et al., 2009). For example, mutant forms of Cx31 can traffic incorrectly and accumulate in organelles such as the ER, causing ER stress and activation of the unfolded protein response (UPR), which results in cell death (Tattersall et al., 2009). Other mutant connexins are able to traffic normally but, on reaching the cell surface, they form leaky hemichannels that are sensitive to low extracellular Ca2+ levels, thus also resulting in increased levels of cell death (Gerido et al., 2007; Lee et al., 2009). Furthermore, over-active hemichannels might lead to increased release of secondary metabolites such as ATP into the extracellular environment, which will activate purinergic cell signalling pathways, resulting in a variety of possible outcomes, including inflammation and cell death (Essenfelder et al., 2004; Baroja-Mazo et al., 2013).
Recently, other channel proteins – the aquaporins (AQPs) and the transient receptor potential (TRP) channels – have also been associated with human disorders of keratinisation (Blaydon et al., 2013; Lin et al., 2012) and will form the focus of this Commentary. As well as briefly reviewing the structure and function of aquaporin and TRP channels, we will summarise their roles in the normal function of the skin barrier and discuss the potential mechanisms underlying the diseases that are associated with mutations in two members of these channel protein families, AQP5 and TRPV3. Dominant gain-of-function mutations in AQP5 and TRPV3 have recently been reported as the underlying cause of diffuse non-epidermolytic palmoplantar keratoderma (NEPPK) and Olmsted syndrome, respectively, two rare congenital hyperkeratotic skin disorders that, in common with the gap-junction-associated skin diseases, feature PPK (Lin et al., 2012; Blaydon et al., 2013). We will highlight how these disease-associated variant channels give insight into non-channel-associated functions of these proteins, such as interactions with proteins of the cell–cell junctions and cytoskeleton, as well as their role in the skin barrier function.
Aquaporins
Aquaporins are a family of integral cell membrane proteins that allow the osmotic movement of water across the cell membrane independently of solute transport (King et al., 2004). To date, thirteen mammalian aquaporin family members have been identified, which can be subdivided into two groups – those that are only permeated by water (aquaporins) and the aquaglyceroporins, which also allow the passage of glycerol and other small solutes. Aquaporins share a common protein fold with six transmembrane α-helices (TMHs) and their amino- and carboxy-termini in the cytosol. Two short half-helices abut in the centre of the membrane to form a virtual seventh TMH that is crucial to the selectivity of the channel (Jung et al., 1994). Human AQP5 has been crystallised as a tetramer, which is considered to be the physiologically relevant form of aquaporin (Horsefield et al., 2008). However, unlike gap junctions, where the molecules pass through the hemichannel formed by the connexin subunits, water molecules move through the channel formed by each of the four aquaporin monomers, rather than through the central pore of the aquaporin tetramer (Jung et al., 1994).
Expression of up to six different aquaporins (AQP1, AQP3, AQP5, AQP7, AQP9 and AQP10) has been shown in various cell types that are found in human skin (Boury-Jamot et al., 2006). Among these, the aquaglyceroporin AQP3 is the most abundantly expressed aquaporin in the keratinocytes of the epidermis (Boury-Jamot et al., 2006). AQP7 is found in adipocytes throughout the body, including the hypodermis of the skin, where it is involved in adipocyte metabolism and the regulation of fat accumulation through glycerol transport (Hara-Chikuma et al., 2005; Hibuse et al., 2005). However, it appears that dendritic cells in the skin also express AQP7 (Hara-Chikuma et al., 2012), where it has been shown to be involved in antigen uptake and the initiation of primary immune responses. Similarly, AQP1 is expressed in dermal fibroblasts and melanocytes in addition to endothelial cells throughout the body (Mobasheri and Marples, 2004; Boury-Jamot et al., 2006). Furthermore, AQP9 and AQP10 mRNA have been detected in keratinocytes that grow in monolayer culture (Sugiyama et al., 2001; Boury-Jamot et al., 2006); however, the function of AQP1, AQP9 and AQP10 in the skin is currently unknown. New insights into the importance of aquaporins in the skin has come from human genetic studies linking AQP5 mutations with a diffuse form of PPK, as discussed below.
AQP5
In contrast to AQP3 and the majority of other AQPs expressed in the skin, AQP5 is the only water-selective aquaporin reported in the epidermis. Until recently, the expression of AQP5 in the skin was believed to be restricted to the sweat gland cells in the dermis, although its role in sweat secretion is not clear (Nejsum et al., 2002; Song et al., 2002; Inoue et al., 2013). However, we have recently reported the first inherited skin disease associated with aquaporin mutations and have shown that AQP5 is expressed throughout the epidermis, albeit at a much lower level than seen in the sweat glands, with a particularly strong localisation at the plasma membrane in keratinocytes of the stratum granulosum of the palmar epidermis (Fig. 1C) (Blaydon et al., 2013). We and others have shown that missense mutations in AQP5 underlie an autosomal dominant form of diffuse NEPPK that is characterised by a diffuse even hyperkeratosis, or thickening of the stratum corneum, over the whole of the palms and soles, with affected areas of skin displaying a white spongy appearance upon exposure to water (Blaydon et al., 2013; Cao et al., 2014).
The disease-associated variant AQP5 proteins appear to retain the ability to traffic to the keratinocyte cell membrane, and we have proposed that the variant AQP5 monomers maintain the capacity to form open channels in the plasma membrane that conduct water and, thus, are likely to represent gain-of-function alleles (Blaydon et al., 2013; Cao et al., 2014). In support of this, AQP5 has been shown to play a role in enhancing microtubule organisation and stability in airway epithelial cells (Sidhaye et al., 2012); likewise, we also see increased microtubule stabilisation in the diffuse NEPPK palm when compared with normal palm skin (Blaydon et al., 2013). The limitation of the diffuse NEPPK patient phenotype to the palmoplantar skin argues for a role for AQP5 that is specific to the palmoplantar skin, as opposed to interfollicular skin. For instance, palmoplantar skin typically has to sustain greater levels of mechanical stress and, hence, might show special adaptations, including a thicker epidermis and possibly stronger cell–cell adhesion.
AQP3
The aquaglyceroporin AQP3, the most abundantly expressed aquaporin in keratinocytes, plays an important role in the transport of water and glycerol in the epidermis. It is localised to the plasma membrane of the lower basal layer (the stratum basale) and spinous layer of the epidermis, but is absent from the upper granular layer of keratinocytes (Sougrat et al., 2002) (Fig. 1C). Since its expression was first reported in the rat epidermis (Frigeri et al., 1995), AQP3 has become the most well-studied aquaporin in mammalian skin. Much of what we know about the role of AQP3 in the epidermis has been gleaned from AQP3-null mice, which display impairments in skin hydration, elasticity, barrier recovery and wound healing functions and show reduced glycerol content in the stratum corneum and epidermis (Hara et al., 2002; Ma et al., 2002). Furthermore, replacing glycerol in AQP3-null mice improves the skin phenotype, illustrating the importance of AQP3-mediated glycerol transport for the normal physiology of the skin (Hara and Verkman, 2003). It is clear that AQP3 plays a role in keratinocyte proliferation in both human and mouse epidermis (Hara-Chikuma and Verkman, 2008b; Nakahigashi et al., 2011) and AQP3-facilitated water transport is important for keratinocyte migration in the mouse (Hara-Chikuma and Verkman, 2008a). Furthermore, despite some debate over a role for AQP3 in keratinocyte differentiation in mice (Bollag et al., 2007; Hara-Chikuma et al., 2009; Kim and Lee, 2010), evidence in human keratinocytes supports a role for AQP3 in the negative regulation of differentiation (and promotion of proliferation) through the downregulation of Notch1, a known mediator of keratinocyte differentiation (Guo et al., 2013).
Although mutations in AQP3 have yet to be identified as the underlying cause of an inherited skin disorder, a number of lines of evidence indicate a role for AQP3 in the skin barrier function. AQP3 has been shown to accumulate with E-cadherin (Nejsum and Nelson, 2007), an important component of cell–cell adherens junctions, and AQP3 knockdown in human keratinocytes resulted in a significant decrease in E-cadherin, as well as β-catenin and γ-catenin, in the plasma membrane fraction, suggesting impaired cell–cell adhesion in association with reduced levels of AQP3 (Kim and Lee, 2010). Furthermore, in AQP3-null mice, delayed biosynthesis of stratum corneum lipids owing to reduced epidermal glycerol content is thought to be the cause of both delayed barrier function recovery [as measured by trans-epidermal water loss (TEWL)] after tape stripping and slower wound healing after punch biopsy (Hara et al., 2002). In keeping with a role for AQP3 in the skin barrier, changes in the levels of AQP3 expression have been found in association with human skin conditions where the epidermal barrier is impaired. For example, increased AQP3 expression is seen in patients with atopic eczema and has been proposed to partially account for the increased water loss and dry skin seen in these patients (Olsson et al., 2006; Nakahigashi et al., 2011), and reduced levels of AQP3 are reported in the lesional and peri-lesional skin of psoriasis patients (Voss et al., 2011; Lee et al., 2012). However, it is not known whether altered AQP3 expression is involved with the pathophysiology of these skin diseases or whether it merely represents secondary changes that occur in response to the disease.
TRP channels
TRP proteins are a superfamily of six-transmembrane-domain proteins that form tetrameric cation-permeable pores with huge variations in ion selectivities, modes of action and physiological roles (Clapham, 2003; Montell, 2005). Specifically, they play a crucial role in the sensory physiology, including mechanosensation and thermosensation in the skin. However, TRP channels also play an important role in many non-excitable cells, including keratinocytes, allowing individual cells to sense changes, such as variations in osmolarity and temperature, in the extracellular environment.
TRPC channels
The TRP channel superfamily can be divided into seven subfamilies: five group 1 TRPs (TRPC, TRPV, TRPM, TRPN and TRPA) and two group 2 subfamilies (TRPP and TRPML) that are grouped based on homology rather than their selectivity or function. A number of the ‘classical’ TRPC subfamily channels (so called as they were the first identified members of the TRP superfamily) are expressed in the epidermis, and there is evidence that the TRPC channels might have a key role in promoting Ca2+-induced keratinocyte differentiation and, hence, barrier formation (Cai et al., 2006; Beck et al., 2008; Müller et al., 2008). Extracellular Ca2+ is crucial for the regulation of the process of keratinocyte differentiation (Sharpe et al., 1989; Pillai et al., 1990), and a Ca2+ gradient is seen in the epidermis, with the lowest concentrations found in the proliferating basal layer and the highest concentrations in the upper differentiating granular layer (Menon et al., 1985). A major Ca2+ entry route into cells is the store-operated route, whereby an increase in the concentration of extracellular Ca2+ is detected and leads to the release of Ca2+ from internal stores, such as the ER and Golgi, within the cell, which in turn activates store-operated calcium (SOC) channels in the plasma membrane, allowing an influx of extracellular Ca2+ and increasing the intracellular Ca2+ concentration to a level required to induce differentiation. TRPC1 and TRPC4 have been demonstrated to act as SOC channels in Ca2+-induced differentiation of human keratinocytes in vitro (Tu et al., 2005; Cai et al., 2006; Beck et al., 2008). Moreover, the importance of TRPC channels in keratinocyte differentiation is highlighted by the finding that activation of TRPC6 is sufficient to induce in vitro keratinocyte differentiation to levels similar to those seen when the cells are stimulated with high concentrations of extracellular Ca2+ (Müller et al., 2008). In addition, the importance of normal Ca2+ homeostasis for the maintenance of the epidermal barrier is highlighted in patients with Darier's disease. In Darier's disease, skin fragility and hyperkeratosis are associated with mutations in the ATP2A2 gene encoding SERCA2, a transmembrane ATPase pump that mediates the uptake of cytosolic Ca2+ into internal ER Ca2+ stores (Sakuntabhai et al., 1999). Both mouse and human keratinocytes with reduced levels of SERCA2 show upregulation of TRPC1 and increased Ca2+ entry (Pani et al., 2006). The mechanism for upregulation of TRPC1 in patients with Darier's disease is not clear; however, the increased expression of TRPC1 in human keratinocytes confers resistance to apoptosis and promotes cell survival in a Ca2+-dependent manner (Pani et al., 2006), indicating a role for TRPC1 in cell survival. A role for TRPC channels in the preservation of the epidermal Ca2+ gradient is likewise underlined in psoriasis. In psoriatic skin, an imbalance in keratinocyte proliferation and differentiation is associated with thickening of the epidermis and a reduced skin barrier function. Psoriatic skin also displays a defect in the epidermal Ca2+ gradient (Menon and Elias, 1991), which might be partly explained by a reduction in the expression of TRPC1, TRPC4 and TRPC6 observed in psoriatic keratinocytes (Leuner et al., 2011); however, the mechanism for downregulation of these TRPC channels in psoriatic skin is unknown.
TRPV channels
The vanilloid subfamily of TRP channels (so named due to activation of TRPV1 by capsaicin, a vanilloid compound) are also implicated in epidermal barrier function. Similar to the TRPC channels discussed above, the highly Ca2+-selective TRPV6 channel also has a crucial role in Ca2+-regulated keratinocyte differentiation. TRPV6 expression is upregulated in the presence of increased levels of extracellular Ca2+ and mediates Ca2+ influx, leading to increased cytoplasmic Ca2+ levels in human keratinocytes, whereas knockdown of TRPV6 in human keratinocytes impairs differentiation in vitro (Lehen'kyi et al., 2007). In addition, TRPV6 knockout mice exhibit impaired stratum corneum formation that is associated with a decrease in epidermal Ca2+ content (Bianco et al., 2007). Furthermore, it has been shown that 1,25-dihydroxyvitamin D3, a key regulator of keratinocyte differentiation, regulates the uptake of Ca2+ by TRPV6 in human keratinocytes (Lehen'kyi et al., 2007).
Keratinocytes also express other members of the vanilloid subfamily of TRP channels, namely TRPV1, TRPV3 and TRPV4. In contrast to other TRP channels discussed here, activation of TRPV1 in keratinocytes appears to suppress cell growth and induce apoptosis and, hence, to have a negative role in epidermal barrier formation and recovery (Denda et al., 2007; Tóth et al., 2011; Yun et al., 2011). Nonetheless, TRPV1-mediated Ca2+ influx might still be necessary for keratinocyte polarity and migration (Graham et al., 2013). TRPV3 is highly expressed in keratinocytes (Peier et al., 2002) and, through its wide range of functions, is purported to be one of the most important TRP channels in the skin (Nilius and Bíró, 2013; Nilius et al., 2014). A key role for TRPV3 in proliferation and differentiation of the epidermis is highlighted in Olmsted syndrome patients harbouring dominantly inherited TRPV3 mutations. These gain-of-function mutations in TRPV3 result in increased levels of keratinocyte apoptosis, leading to the formation of hyperkeratotic plaques (Lin et al., 2012). Although skin barrier integrity has not been measured in Olmsted syndrome patients, embryonic day 17 TRPV3-null mouse embryos show significant dye permeability compared with control embryos, indicating a defect in barrier integrity in the absence of TRPV3 in the mouse (Cheng et al., 2010). Also in mice, TRPV3 has been shown to regulate epidermal growth factor receptor (EGFR) signalling as well as the activity of transglutaminases, enzymes that crosslink proteins during the formation of the cornified envelope (Cheng et al., 2010). The EGFR signalling pathway is important for the balance between keratinocyte proliferation and differentiation (Schneider et al., 2008) and, in TRPV3-null mouse skin, the level of EGFR activity is reduced (Cheng et al., 2010). Furthermore, TRPV3-null mice also exhibit a ‘wavy’ hair phenotype (Cheng et al., 2010), similar to transforming growth factor-α (TGFα) and EGFR-knockout mice (Luetteke et al., 1993; Mann et al., 1993), supporting the link with EGFR signalling. By contrast, Olmsted syndrome patients with activating TRPV3 mutations exhibit diffuse alopecia, and mice carrying gain-of-function TRPV3 mutations are hairless (Asakawa et al., 2006; Xiao et al., 2008). Moreover, activation of TRPV3 in human hair follicle culture causes inhibition of hair shaft elongation and apoptosis-driven catagen formation (Borbíró et al., 2011).
Therefore, TRPV3 appears to have a crucial role in the formation of the stratum corneum, by means of regulation of the differentiation process. However, it has emerged that TRPV4, a TRP channel closely related to TRPV3, is involved in the formation of the intercellular junctions (tight junctions and adherens junctions) between cells that are located just below the stratum corneum and that form another essential component of the epidermal barrier (Niessen, 2007; Kirschner and Brandner, 2012). TRPV4 interacts with β-catenin and E-cadherin, and TRPV4 activation strengthens the tight junction barrier and accelerates barrier recovery (Sokabe et al., 2010; Kida et al., 2012; Akazawa et al., 2013). TRPV4 is also activated by changes in osmolarity (Liedtke et al., 2000), and it has been suggested that TRPV4 might act as a sensor of humidity or water flux from the skin surface as part of the epidermal permeability barrier homeostasis (Denda et al., 2007). TRPV4 belongs to a group of temperature-sensitive TRP channels known as thermoTRPs, and it is activated by temperatures in the physiological skin temperature range (around 33°C) (Chung et al., 2004; Lee and Caterina, 2005); consequently, TRPV4-null mice display abnormal thermosensation behaviour (Lee and Caterina, 2005). Warmth activation of TRPV4 leads to a Ca2+ influx through the channels, and the increase in intracellular Ca2+ concentration promotes Rho activation, which leads to the reorganisation of the actin cytoskeleton and enhanced integrity of the intercellular junctions and, hence, enhanced barrier function (Sokabe et al., 2010). Therefore, it appears that keratinocytes might have a central role in thermosensation of the skin that is mediated, at least in part, by TRP channels.
Interestingly, there appears to be an association between TRPV4 and AQP5 in certain cell types. TRPV4 and AQP5 are both expressed at sites where extracellular osmolarity is known to fluctuate (e.g. in the respiratory epithelium, salivary glands and sweat glands) and a decrease in the abundance of AQP5 at the cell surface in response to hypotonic conditions is regulated by TRPV4 activation in lung epithelial cells (Sidhaye et al., 2006) and salivary gland cells (Liu et al., 2006). Furthermore, regulatory volume decrease (RVD), a mechanism that allows cells to recover their original volume in the presence of osmotic stress, appears to be controlled in salivary gland cells by the interaction of TRPV4 and AQP5 (Liu et al., 2006). In addition, activation of TRPV4 also decreases the abundance of AQP5 at the cell membrane and enhances epithelial barrier integrity in response to shear stress in airway epithelial cells (Sidhaye et al., 2008), and loss of AQP5 is associated with a decrease in paracellular permeability in mice salivary glands (Kawedia et al., 2007). It is possible that AQP5 and TRPV4 might have a similar relationship in keratinocytes. AQP5 and TRPV4 proteins are both expressed in the upper layers of the palm epidermis (Fig. 1C), and diffuse NEPPK patients with gain-of-function AQP5 mutations exhibit increased microtubule stabilisation (Blaydon et al., 2013), which, at least in human airway epithelial cells, is a non-channel function of AQP5 that has been associated with increased paracellular permeability (Sidhaye et al., 2008; Sidhaye et al., 2012). Furthermore, in an in vitro patch clamp assay, the basal activity of TRPV4 was increased in the presence of mutant AQP5 compared with the activity seen with wild-type AQP5 (Cao et al., 2014). Therefore, AQP5 and TRPV4 might have a similar interaction in keratinocytes for detecting and responding to osmotic and mechanical stress by modulating the epidermal barrier, in particular through alterations in cell–cell junctions, such as the tight junctions (Fig. 2). Consequently, it is possible that the gain-of-function AQP5 alleles that are associated with diffuse NEPPK might remain present at the plasma membrane inappropriately and result in impaired barrier integrity, possibly through increased paracellular permeability; however, further studies are required to confirm this.
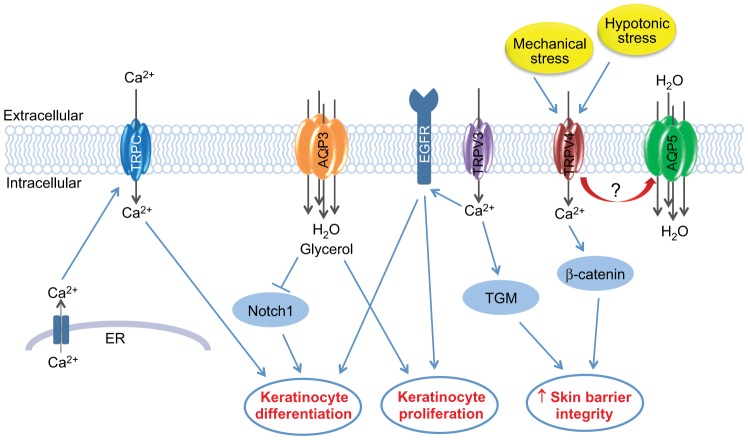
Fig. 2.
Illustration of the role of key aquaporin and TRP channel proteins in formation of the epidermal barrier. TRP channels form tetrameric cation-permeable pores, which allow the movement of a variety of cations, including Ca2+, which is crucial for the regulation of keratinocyte differentiation. A number of TRPC channels (blue) are expressed in keratinocytes, and there is evidence supporting a key role for these channels in the promotion of Ca2+-induced keratinocyte differentiation. TRPC channels are believed to be activated (thus allowing the influx of extracellular Ca2+) upon detection of Ca2+ release from internal stores, such as the ER. TRPV3 (purple) appears to play a central role in keratinocyte biology, with a key role in establishing the balance between keratinocyte proliferation and differentiation, which is believed to be mediated through EGFR activation. TRPV3 might also have a more direct role in the formation of the epidermal barrier through regulation of the activity of transglutaminases (TGM), the enzymes required for the cross-linking of protein components of the cornified envelope. Furthermore, TRPV4 (red), which is activated in response to mechanical and hypotonic stress, has been shown to bind to components of the intercellular junctions, such as β-catenin, leading to increased skin barrier integrity. AQP3 (orange), an aquaglyceroporin that is abundantly expressed in keratinocytes, also allows the movement of small molecules, such as glycerol, in addition to water, into keratinocytes. AQP3 appears to play a central role in keratinocyte proliferation, and although the role for AQP3 in keratinocyte differentiation is less clear, there is evidence that it might be involved in the negative regulation of differentiation through inhibition of Notch1, a known mediator of keratinocyte differentiation. AQP5 is a water-selective aquaporin, the role of which in the formation of the epidermal barrier is currently unclear. However, AQP5 (green) might have a role in the regulation of cell–cell adhesion, either through microtubule stabilisation or through an association with TRPV4, as has been shown in other cell types, but further work is required to determine this.
Therefore, the aquaporin and TRP channel proteins are emerging as central players in the formation and maintenance of skin barrier function, and they act through a variety of different mechanisms, some of which are non-channel-associated functions (Fig. 2).
Perspectives
A common theme emerging amongst these three families of channels in the skin – gap junctions, aquaporins and TRP channels – is that the human epidermal barrier appears to be capable of tolerating loss-of-function mutations in these channels, probably due to a level of redundancy between members of these protein families in the skin, whereas gain-of-function mutations are associated with an impaired skin barrier. This is particularly clear with mutations in connexins, whereby recessive loss-of-function mutations underlie non-syndromic deafness in the absence of an associated skin phenotype (Scott and Kelsell, 2011). This might also be the case for AQP5, as AQP5-null mice have no reported skin abnormalities (Song et al., 2002), although the anatomy of the skin has not been directly studied in these mice. However, mice lacking TRPV3 expression in keratinocytes exhibit a significantly thinner stratum corneum, altered keratinocyte differentiation and impaired barrier function (Cheng et al., 2010), whereas AQP3-null mice retain normal barrier function, but display delayed barrier recovery after disruption (Hara et al., 2002). It remains to be seen whether the loss of functional AQP5 and TRPV3 has an effect on epidermal barrier function in human skin and whether any AQP3 variants will be associated with a human skin phenotype in the future.
It is intriguing that mutations in these channel proteins are all associated with hyperkeratotic skin diseases that affect the palms and soles (PPK) in particular. This might indicate that the balance between proliferation and differentiation in the formation and maintenance of the epidermal barrier in palmoplantar skin is particularly sensitive to alterations in the normal physiology and highlight a key role for channel proteins in the skin barrier function. For example, increased cell death leading to barrier dysfunction is seen in association with gain-of-function connexin variants in EKV and KID syndrome, as well as in association with TRPV3 variants in Olmsted patients. Furthermore, these mutant channel proteins give us insight into important roles for alternative non-channel functions for these proteins, particularly in the skin barrier. For example, we see increased microtubule stabilisation in association with gain-of-function variants of AQP5; however, further studies are required to understand the mechanism of microtubule stabilisation by AQP5 and to unravel the downstream effects of this alteration in the cell cytoskeleton. There is also evidence for interplay between these channels; in particular this has been shown for AQP5 and TRPV4 in other cell types, but it remains to be confirmed in keratinocytes.
Therefore, although there is clear genetic evidence that channel proteins have a key role in the normal biology of keratinocytes, the mechanisms through which these channel proteins act in the formation, maintenance and regeneration of the human epidermal barrier still need to be clearly defined. A valuable tool for unravelling these mechanisms are 3D skin models that mimic key features of the skin barrier function in vitro (Frankart et al., 2012) and which can be used for further dissection of normal and mutant channel proteins using both patient-derived keratinocytes and normal cells that have been genetically manipulated. These in vitro disease models can also be used to optimise therapies for these skin conditions, such as topical barrier creams containing small molecule inhibitors specific for the channel protein of interest. Alternatively, allele-specific small interfering (si)RNA-mediated knockdown of the variant channel, such as is being developed for some of the rare monogenic keratin disorders (McLean and Moore, 2011), provides a more targeted approach that leaves the wild-type channel intact. A host of other diverse protein channels are also expressed in the skin that have important roles in a variety of functions, including barrier homeostasis, the immunological barrier, barrier regeneration and sensory functions (e.g. touch, pain and thermoregulation) (Oláh et al., 2012). For example, ENaC, an epithelial sodium channel with a key role in regulating the transport of sodium ions in electrically tight epithelia such as the renal collecting duct (Garty and Palmer, 1997), is also expressed in the epidermis and cultured keratinocytes (Brouard et al., 1999), and appears to be required for the directional migration of keratinocytes in an electric field, indicating a role for ENaC in wound healing (Yang et al., 2013). Utilising next-generation sequencing technologies, numerous new genes that are involved in inherited skin diseases are being identified. It is likely that further channel or channel-associated proteins will be implicated in keratinocyte biology and, with the visible and accessible nature of the skin, their functions and disease-associated mechanisms are likely to be revealed.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing interests.
Funding
D.P.K. is funded by the Medical Research Council; Barts and the London Charity; and the British Heart Foundation.
References
- Ahmad S., Diez J. A., George C. H., Evans W. H. (1999). Synthesis and assembly of connexins in vitro into homomeric and heteromeric functional gap junction hemichannels. Biochem. J. 339, 247–253 10.1042/0264-6021:3390247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akazawa Y., Yuki T., Yoshida H., Sugiyama Y., Inoue S. (2013). Activation of TRPV4 strengthens the tight-junction barrier in human epidermal keratinocytes. Skin Pharmacol. Physiol. 26, 15–21 10.1159/000343173 [DOI] [PubMed] [Google Scholar]
- Asakawa M., Yoshioka T., Matsutani T., Hikita I., Suzuki M., Oshima I., Tsukahara K., Arimura A., Horikawa T., Hirasawa T. et al. (2006). Association of a mutation in TRPV3 with defective hair growth in rodents. J. Invest. Dermatol. 126, 2664–2672 10.1038/sj.jid.5700468 [DOI] [PubMed] [Google Scholar]
- Bakirtzis G., Choudhry R., Aasen T., Shore L., Brown K., Bryson S., Forrow S., Tetley L., Finbow M., Greenhalgh D. et al. (2003). Targeted epidermal expression of mutant Connexin 26(D66H) mimics true Vohwinkel syndrome and provides a model for the pathogenesis of dominant connexin disorders. Hum. Mol. Genet. 12, 1737–1744 10.1093/hmg/ddg183 [DOI] [PubMed] [Google Scholar]
- Baroja-Mazo A., Barberà-Cremades M., Pelegrín P. (2013). The participation of plasma membrane hemichannels to purinergic signaling. Biochim. Biophys. Acta 1828, 79–93 10.1016/j.bbamem.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Beck B., Lehen'kyi V., Roudbaraki M., Flourakis M., Charveron M., Bordat P., Polakowska R., Prevarskaya N., Skryma R. (2008). TRPC channels determine human keratinocyte differentiation: new insight into basal cell carcinoma. Cell Calcium 43, 492–505 10.1016/j.ceca.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Bianco S. D., Peng J. B., Takanaga H., Suzuki Y., Crescenzi A., Kos C. H., Zhuang L., Freeman M. R., Gouveia C. H., Wu J. et al. (2007). Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J. Bone Miner. Res. 22, 274–285 10.1359/jbmr.061110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaydon D. C., Lind L. K., Plagnol V., Linton K. J., Smith F. J., Wilson N. J., McLean W. H., Munro C. S., South A. P., Leigh I. M. et al. (2013). Mutations in AQP5, encoding a water-channel protein, cause autosomal-dominant diffuse nonepidermolytic palmoplantar keratoderma. Am. J. Hum. Genet. 93, 330–335 10.1016/j.ajhg.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag W. B., Xie D., Zheng X., Zhong X. (2007). A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: production of a phosphatidylglycerol signaling lipid. J. Invest. Dermatol. 127, 2823–2831 [DOI] [PubMed] [Google Scholar]
- Borbíró I., Lisztes E., Tóth B. I., Czifra G., Oláh A., Szöllosi A. G., Szentandrássy N., Nánási P. P., Péter Z., Paus R. et al. (2011). Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J. Invest. Dermatol. 131, 1605–1614 10.1038/jid.2011.122 [DOI] [PubMed] [Google Scholar]
- Boury-Jamot M., Sougrat R., Tailhardat M., Le Varlet B., Bonté F., Dumas M., Verbavatz J. M. (2006). Expression and function of aquaporins in human skin: Is aquaporin-3 just a glycerol transporter? Biochim. Biophys. Acta 1758, 1034–1042 10.1016/j.bbamem.2006.06.013 [DOI] [PubMed] [Google Scholar]
- Brouard M., Casado M., Djelidi S., Barrandon Y., Farman N. (1999). Epithelial sodium channel in human epidermal keratinocytes: expression of its subunits and relation to sodium transport and differentiation. J. Cell Sci. 112, 3343–3352 [DOI] [PubMed] [Google Scholar]
- Cai S., Fatherazi S., Presland R. B., Belton C. M., Roberts F. A., Goodwin P. C., Schubert M. M., Izutsu K. T. (2006). Evidence that TRPC1 contributes to calcium-induced differentiation of human keratinocytes. Pflugers Arch. 452, 43–52 10.1007/s00424-005-0001-1 [DOI] [PubMed] [Google Scholar]
- Cao X., Yin J., Wang H., Zhao J., Zhang J., Dai L., Zhang J., Jiang H., Lin Z., Yang Y. (2014). Mutation in AQP5, encoding aquaporin 5, causes palmoplantar keratoderma Bothnia type. J. Invest. Dermatol. 134, 284–287 10.1038/jid.2013.302 [DOI] [PubMed] [Google Scholar]
- Caspar D. L., Goodenough D. A., Makowski L., Phillips W. C. (1977). Gap junction structures. I. Correlated electron microscopy and x-ray diffraction. J. Cell Biol. 74, 605–628 10.1083/jcb.74.2.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Jin J., Hu L., Shen D., Dong X. P., Samie M. A., Knoff J., Eisinger B., Liu M. L., Huang S. M. et al. (2010). TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 141, 331–343 10.1016/j.cell.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M. K., Lee H., Mizuno A., Suzuki M., Caterina M. J. (2004). TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J. Biol. Chem. 279, 21569–21575 10.1074/jbc.M401872200 [DOI] [PubMed] [Google Scholar]
- Clapham D. E. (2003). TRP channels as cellular sensors. Nature 426, 517–524 10.1038/nature02196 [DOI] [PubMed] [Google Scholar]
- Coutinho P., Qiu C., Frank S., Tamber K., Becker D. (2003). Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell Biol. Int. 27, 525–541 10.1016/S1065-6995(03)00077-5 [DOI] [PubMed] [Google Scholar]
- D'Adamo P., Guerci V. I., Fabretto A., Faletra F., Grasso D. L., Ronfani L., Montico M., Morgutti M., Guastalla P., Gasparini P. (2009). Does epidermal thickening explain GJB2 high carrier frequency and heterozygote advantage? Eur. J. Hum. Genet. 17, 284–286 10.1038/ejhg.2008.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denda M., Sokabe T., Fukumi-Tominaga T., Tominaga M. (2007). Effects of skin surface temperature on epidermal permeability barrier homeostasis. J. Invest. Dermatol. 127, 654–659 10.1038/sj.jid.5700590 [DOI] [PubMed] [Google Scholar]
- Di W. L., Rugg E. L., Leigh I. M., Kelsell D. P. (2001). Multiple epidermal connexins are expressed in different keratinocyte subpopulations including connexin 31. J. Invest. Dermatol. 117, 958–964 10.1046/j.0022-202x.2001.01468.x [DOI] [PubMed] [Google Scholar]
- Djalilian A. R., McGaughey D., Patel S., Seo E. Y., Yang C., Cheng J., Tomic M., Sinha S., Ishida-Yamamoto A., Segre J. A. (2006). Connexin 26 regulates epidermal barrier and wound remodeling and promotes psoriasiform response. J. Clin. Invest. 116, 1243–1253 10.1172/JCI27186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essenfelder G. M., Bruzzone R., Lamartine J., Charollais A., Blanchet-Bardon C., Barbe M. T., Meda P., Waksman G. (2004). Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum. Mol. Genet. 13, 1703–1714 10.1093/hmg/ddh191 [DOI] [PubMed] [Google Scholar]
- Frankart A., Malaisse J., De Vuyst E., Minner F., de Rouvroit C. L., Poumay Y. (2012). Epidermal morphogenesis during progressive in vitro 3D reconstruction at the air-liquid interface. Exp. Dermatol. 21, 871–875 10.1111/exd.12020 [DOI] [PubMed] [Google Scholar]
- Frigeri A., Gropper M. A., Umenishi F., Kawashima M., Brown D., Verkman A. S. (1995). Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J. Cell Sci. 108, 2993–3002 [DOI] [PubMed] [Google Scholar]
- Garty H., Palmer L. G. (1997). Epithelial sodium channels: function, structure, and regulation. Physiol. Rev. 77, 359–396 [DOI] [PubMed] [Google Scholar]
- Gerido D. A., DeRosa A. M., Richard G., White T. W. (2007). Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am. J. Physiol. 293, C337–C345 10.1152/ajpcell.00626.2006 [DOI] [PubMed] [Google Scholar]
- Goliger J. A., Paul D. L. (1995). Wounding alters epidermal connexin expression and gap junction-mediated intercellular communication. Mol. Biol. Cell 6, 1491–1501 10.1091/mbc.6.11.1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. M., Huang L., Robinson K. R., Messerli M. A. (2013). Epidermal keratinocyte polarity and motility require Ca2+ influx through TRPV1. J. Cell Sci. 126, 4602–4613 10.1242/jcs.122192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastalla P., Guerci V. I., Fabretto A., Faletra F., Grasso D. L., Zocconi E., Stefanidou D., D'Adamo P., Ronfani L., Montico M. et al. (2009). Detection of epidermal thickening in GJB2 carriers with epidermal US. Radiology 251, 280–286 10.1148/radiol.2511080912 [DOI] [PubMed] [Google Scholar]
- Guo L., Chen H., Li Y., Zhou Q., Sui Y. (2013). An aquaporin 3-notch1 axis in keratinocyte differentiation and inflammation. PLoS ONE 8, e80179 10.1371/journal.pone.0080179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M., Verkman A. S. (2003). Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc. Natl. Acad. Sci. USA 100, 7360–7365 10.1073/pnas.1230416100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M., Ma T., Verkman A. S. (2002). Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J. Biol. Chem. 277, 46616–46621 10.1074/jbc.M209003200 [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M., Verkman A. S. (2008a). Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J. Mol. Med. (Berl) 86, 221–231 10.1007/s00109-007-0272-4 [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M., Verkman A. S. (2008b). Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol. Cell. Biol. 28, 326–332 10.1128/MCB.01482-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M., Sohara E., Rai T., Ikawa M., Okabe M., Sasaki S., Uchida S., Verkman A. S. (2005). Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation. J. Biol. Chem. 280, 15493–15496 10.1074/jbc.C500028200 [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M., Takahashi K., Chikuma S., Verkman A. S., Miyachi Y. (2009). The expression of differentiation markers in aquaporin-3 deficient epidermis. Arch. Dermatol. Res. 301, 245–252 10.1007/s00403-009-0927-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M., Sugiyama Y., Kabashima K., Sohara E., Uchida S., Sasaki S., Inoue S., Miyachi Y. (2012). Involvement of aquaporin-7 in the cutaneous primary immune response through modulation of antigen uptake and migration in dendritic cells. FASEB J. 26, 211–218 10.1096/fj.11-186627 [DOI] [PubMed] [Google Scholar]
- Hibuse T., Maeda N., Funahashi T., Yamamoto K., Nagasawa A., Mizunoya W., Kishida K., Inoue K., Kuriyama H., Nakamura T. et al. (2005). Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc. Natl. Acad. Sci. USA 102, 10993–10998 10.1073/pnas.0503291102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsefield R., Nordén K., Fellert M., Backmark A., Törnroth-Horsefield S., Terwisscha van Scheltinga A. C., Kvassman J., Kjellbom P., Johanson U., Neutze R. (2008). High-resolution x-ray structure of human aquaporin 5. Proc. Natl. Acad. Sci. USA 105, 13327–13332 10.1073/pnas.0801466105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Sohara E., Rai T., Satoh T., Yokozeki H., Sasaki S., Uchida S. (2013). Immunolocalization and translocation of aquaporin-5 water channel in sweat glands. J. Dermatol. Sci. 70, 26–33 10.1016/j.jdermsci.2013.01.013 [DOI] [PubMed] [Google Scholar]
- Jung J. S., Preston G. M., Smith B. L., Guggino W. B., Agre P. (1994). Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J. Biol. Chem. 269, 14648–14654 [PubMed] [Google Scholar]
- Kandyba E. E., Hodgins M. B., Martin P. E. (2008). A murine living skin equivalent amenable to live-cell imaging: analysis of the roles of connexins in the epidermis. J. Invest. Dermatol. 128, 1039–1049 10.1038/sj.jid.5701125 [DOI] [PubMed] [Google Scholar]
- Kawedia J. D., Nieman M. L., Boivin G. P., Melvin J. E., Kikuchi K., Hand A. R., Lorenz J. N., Menon A. G. (2007). Interaction between transcellular and paracellular water transport pathways through Aquaporin 5 and the tight junction complex. Proc. Natl. Acad. Sci. USA 104, 3621–3626 10.1073/pnas.0608384104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida N., Sokabe T., Kashio M., Haruna K., Mizuno Y., Suga Y., Nishikawa K., Kanamaru A., Hongo M., Oba A. et al. (2012). Importance of transient receptor potential vanilloid 4 (TRPV4) in epidermal barrier function in human skin keratinocytes. Pflugers Arch. 463, 715–725 10.1007/s00424-012-1081-3 [DOI] [PubMed] [Google Scholar]
- Kim N. H., Lee A. Y. (2010). Reduced aquaporin3 expression and survival of keratinocytes in the depigmented epidermis of vitiligo. J. Invest. Dermatol. 130, 2231–2239 10.1038/jid.2010.99 [DOI] [PubMed] [Google Scholar]
- King L. S., Kozono D., Agre P. (2004). From structure to disease: the evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 5, 687–698 10.1038/nrm1469 [DOI] [PubMed] [Google Scholar]
- Kirschner N., Brandner J. M. (2012). Barriers and more: functions of tight junction proteins in the skin. Ann. N. Y. Acad. Sci. 1257, 158–166 10.1111/j.1749-6632.2012.06554.x [DOI] [PubMed] [Google Scholar]
- Lee H., Caterina M. J. (2005). TRPV channels as thermosensory receptors in epithelial cells. Pflugers Arch. 451, 160–167 10.1007/s00424-005-1438-y [DOI] [PubMed] [Google Scholar]
- Lee J. R., Derosa A. M., White T. W. (2009). Connexin mutations causing skin disease and deafness increase hemichannel activity and cell death when expressed in Xenopus oocytes. J. Invest. Dermatol. 129, 870–878 10.1038/jid.2008.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Je Y. J., Lee S. S., Li Z. J., Choi D. K., Kwon Y. B., Sohn K. C., Im M., Seo Y. J., Lee J. H. (2012). Changes in transepidermal water loss and skin hydration according to expression of aquaporin-3 in psoriasis. Ann. Dermatol 24, 168–174 10.5021/ad.2012.24.2.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehen'kyi V., Beck B., Polakowska R., Charveron M., Bordat P., Skryma R., Prevarskaya N. (2007). TRPV6 is a Ca2+ entry channel essential for Ca2+-induced differentiation of human keratinocytes. J. Biol. Chem. 282, 22582–22591 10.1074/jbc.M611398200 [DOI] [PubMed] [Google Scholar]
- Leuner K., Kraus M., Woelfle U., Beschmann H., Harteneck C., Boehncke W. H., Schempp C. M., Müller W. E. (2011). Reduced TRPC channel expression in psoriatic keratinocytes is associated with impaired differentiation and enhanced proliferation. PLoS ONE 6, e14716 10.1371/journal.pone.0014716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W., Choe Y., Martí-Renom M. A., Bell A. M., Denis C. S., Sali A., Hudspeth A. J., Friedman J. M., Heller S. (2000). Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103, 525–535 10.1016/S0092-8674(00)00143-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z., Chen Q., Lee M., Cao X., Zhang J., Ma D., Chen L., Hu X., Wang H., Wang X. et al. (2012). Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am. J. Hum. Genet. 90, 558–564 10.1016/j.ajhg.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Bandyopadhyay B. C., Nakamoto T., Singh B., Liedtke W., Melvin J. E., Ambudkar I. (2006). A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J. Biol. Chem. 281, 15485–15495 10.1074/jbc.M600549200 [DOI] [PubMed] [Google Scholar]
- Luetteke N. C., Qiu T. H., Peiffer R. L., Oliver P., Smithies O., Lee D. C. (1993). TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell 73, 263–278 10.1016/0092-8674(93)90228-I [DOI] [PubMed] [Google Scholar]
- Ma T., Hara M., Sougrat R., Verbavatz J. M., Verkman A. S. (2002). Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J. Biol. Chem. 277, 17147–17153 10.1074/jbc.M200925200 [DOI] [PubMed] [Google Scholar]
- Man Y. K., Trolove C., Tattersall D., Thomas A. C., Papakonstantinopoulou A., Patel D., Scott C., Chong J., Jagger D. J., O'Toole E. A. et al. (2007). A deafness-associated mutant human connexin 26 improves the epithelial barrier in vitro. J. Membr. Biol. 218, 29–37 10.1007/s00232-007-9025-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann G. B., Fowler K. J., Gabriel A., Nice E. C., Williams R. L., Dunn A. R. (1993). Mice with a null mutation of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell 73, 249–261 10.1016/0092-8674(93)90227-H [DOI] [PubMed] [Google Scholar]
- Martin P. E., Blundell G., Ahmad S., Errington R. J., Evans W. H. (2001). Multiple pathways in the trafficking and assembly of connexin 26, 32 and 43 into gap junction intercellular communication channels. J. Cell Sci. 114, 3845–3855 [DOI] [PubMed] [Google Scholar]
- Martin P. E., Easton J. A., Hodgins M. B., Wright C. S. (2014). Connexins: sensors of epidermal integrity that are therapeutic targets. FEBS Lett. 588, 1304–1314 10.1016/j.febslet.2014.02.048 [DOI] [PubMed] [Google Scholar]
- McLean W. H., Moore C. B. (2011). Keratin disorders: from gene to therapy. Hum. Mol. Genet. 20 R2, R189–R197 10.1093/hmg/ddr379 [DOI] [PubMed] [Google Scholar]
- Menon G. K., Elias P. M. (1991). Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch. Dermatol. 127, 57–63 10.1001/archderm.1991.01680010067010 [DOI] [PubMed] [Google Scholar]
- Menon G. K., Grayson S., Elias P. M. (1985). Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J. Invest. Dermatol. 84, 508–512 10.1111/1523-1747.ep12273485 [DOI] [PubMed] [Google Scholar]
- Mobasheri A., Marples D. (2004). Expression of the AQP-1 water channel in normal human tissues: a semiquantitative study using tissue microarray technology. Am. J. Physiol. 286, C529–C537 10.1152/ajpcell.00408.2003 [DOI] [PubMed] [Google Scholar]
- Montell C. (2005). The TRP superfamily of cation channels. Sci. STKE 2005, re3. [DOI] [PubMed] [Google Scholar]
- Mori R., Power K. T., Wang C. M., Martin P., Becker D. L. (2006). Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J. Cell Sci. 119, 5193–5203 10.1242/jcs.03320 [DOI] [PubMed] [Google Scholar]
- Müller M., Essin K., Hill K., Beschmann H., Rubant S., Schempp C. M., Gollasch M., Boehncke W. H., Harteneck C., Müller W. E. et al. (2008). Specific TRPC6 channel activation, a novel approach to stimulate keratinocyte differentiation. J. Biol. Chem. 283, 33942–33954 10.1074/jbc.M801844200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahigashi K., Kabashima K., Ikoma A., Verkman A. S., Miyachi Y., Hara-Chikuma M. (2011). Upregulation of aquaporin-3 is involved in keratinocyte proliferation and epidermal hyperplasia. J. Invest. Dermatol. 131, 865–873 10.1038/jid.2010.395 [DOI] [PubMed] [Google Scholar]
- Nejsum L. N., Nelson W. J. (2007). A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J. Cell Biol. 178, 323–335 10.1083/jcb.200705094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejsum L. N., Kwon T. H., Jensen U. B., Fumagalli O., Frøkiaer J., Krane C. M., Menon A. G., King L. S., Agre P. C., Nielsen S. (2002). Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc. Natl. Acad. Sci. USA 99, 511–516 10.1073/pnas.012588099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen C. M. (2007). Tight junctions/adherens junctions: basic structure and function. J. Invest. Dermatol. 127, 2525–2532 10.1038/sj.jid.5700865 [DOI] [PubMed] [Google Scholar]
- Nilius B., Bíró T. (2013). TRPV3: a ‘more than skinny’ channel. Exp. Dermatol. 22, 447–452 10.1111/exd.12163 [DOI] [PubMed] [Google Scholar]
- Nilius B., Bíró T., Owsianik G. (2014). TRPV3: time to decipher a poorly understood family member! J. Physiol. 592, 295–304 10.1113/jphysiol.2013.255968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláh A., Szöllo˝si A. G., Bíró T. (2012). The channel physiology of the skin. Rev. Physiol. Biochem. Pharmacol. 163, 65–131 [DOI] [PubMed] [Google Scholar]
- Olsson M., Broberg A., Jernås M., Carlsson L., Rudemo M., Suurküla M., Svensson P. A., Benson M. (2006). Increased expression of aquaporin 3 in atopic eczema. Allergy 61, 1132–1137 10.1111/j.1398-9995.2006.01151.x [DOI] [PubMed] [Google Scholar]
- Pani B., Cornatzer E., Cornatzer W., Shin D. M., Pittelkow M. R., Hovnanian A., Ambudkar I. S., Singh B. B. (2006). Up-regulation of transient receptor potential canonical 1 (TRPC1) following sarco(endo)plasmic reticulum Ca2+ ATPase 2 gene silencing promotes cell survival: a potential role for TRPC1 in Darier's disease. Mol. Biol. Cell 17, 4446–4458 10.1091/mbc.E06-03-0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier A. M., Reeve A. J., Andersson D. A., Moqrich A., Earley T. J., Hergarden A. C., Story G. M., Colley S., Hogenesch J. B., McIntyre P. et al. (2002). A heat-sensitive TRP channel expressed in keratinocytes. Science 296, 2046–2049 10.1126/science.1073140 [DOI] [PubMed] [Google Scholar]
- Pillai S., Bikle D. D., Mancianti M. L., Cline P., Hincenbergs M. (1990). Calcium regulation of growth and differentiation of normal human keratinocytes: modulation of differentiation competence by stages of growth and extracellular calcium. J. Cell. Physiol. 143, 294–302 10.1002/jcp.1041430213 [DOI] [PubMed] [Google Scholar]
- Proksch E., Brandner J. M., Jensen J. M. (2008). The skin: an indispensable barrier. Exp. Dermatol. 17, 1063–1072 10.1111/j.1600-0625.2008.00786.x [DOI] [PubMed] [Google Scholar]
- Qiu C., Coutinho P., Frank S., Franke S., Law L. Y., Martin P., Green C. R., Becker D. L. (2003). Targeting connexin43 expression accelerates the rate of wound repair. Curr. Biol. 13, 1697–1703 10.1016/j.cub.2003.09.007 [DOI] [PubMed] [Google Scholar]
- Richard G., White T. W., Smith L. E., Bailey R. A., Compton J. G., Paul D. L., Bale S. J. (1998). Functional defects of Cx26 resulting from a heterozygous missense mutation in a family with dominant deaf-mutism and palmoplantar keratoderma. Hum. Genet. 103, 393–399 10.1007/s004390050839 [DOI] [PubMed] [Google Scholar]
- Rouan F., White T. W., Brown N., Taylor A. M., Lucke T. W., Paul D. L., Munro C. S., Uitto J., Hodgins M. B., Richard G. (2001). trans-dominant inhibition of connexin-43 by mutant connexin-26: implications for dominant connexin disorders affecting epidermal differentiation. J. Cell Sci. 114, 2105–2113 [DOI] [PubMed] [Google Scholar]
- Sakuntabhai A., Ruiz-Perez V., Carter S., Jacobsen N., Burge S., Monk S., Smith M., Munro C. S., O'Donovan M., Craddock N. et al. (1999). Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat. Genet. 21, 271–277 10.1038/6784 [DOI] [PubMed] [Google Scholar]
- Schneider M. R., Werner S., Paus R., Wolf E. (2008). Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. Am. J. Pathol. 173, 14–24 10.2353/ajpath.2008.070942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. A., Kelsell D. P. (2011). Key functions for gap junctions in skin and hearing. Biochem. J. 438, 245–254 10.1042/BJ20110278 [DOI] [PubMed] [Google Scholar]
- Sharpe G. R., Gillespie J. I., Greenwell J. R. (1989). An increase in intracellular free calcium is an early event during differentiation of cultured human keratinocytes. FEBS Lett. 254, 25–28 10.1016/0014-5793(89)81002-6 [DOI] [PubMed] [Google Scholar]
- Sidhaye V. K., Güler A. D., Schweitzer K. S., D'Alessio F., Caterina M. J., King L. S. (2006). Transient receptor potential vanilloid 4 regulates aquaporin-5 abundance under hypotonic conditions. Proc. Natl. Acad. Sci. USA 103, 4747–4752 10.1073/pnas.0511211103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhaye V. K., Schweitzer K. S., Caterina M. J., Shimoda L., King L. S. (2008). Shear stress regulates aquaporin-5 and airway epithelial barrier function. Proc. Natl. Acad. Sci. USA 105, 3345–3350 10.1073/pnas.0712287105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhaye V. K., Chau E., Srivastava V., Sirimalle S., Balabhadrapatruni C., Aggarwal N. R., D'Alessio F. R., Robinson D. N., King L. S. (2012). A novel role for aquaporin-5 in enhancing microtubule organization and stability. PLoS ONE 7, e38717 10.1371/journal.pone.0038717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C., Kelsell D. P., Marchès O. (2013). Connexin 26 facilitates gastrointestinal bacterial infection in vitro. Cell Tissue Res. 351, 107–116 10.1007/s00441-012-1502-9 [DOI] [PubMed] [Google Scholar]
- Sokabe T., Fukumi-Tominaga T., Yonemura S., Mizuno A., Tominaga M. (2010). The TRPV4 channel contributes to intercellular junction formation in keratinocytes. J. Biol. Chem. 285, 18749–18758 10.1074/jbc.M110.103606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Sonawane N., Verkman A. S. (2002). Localization of aquaporin-5 in sweat glands and functional analysis using knockout mice. J. Physiol. 541, 561–568 10.1113/jphysiol.2001.020180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sougrat R., Morand M., Gondran C., Barré P., Gobin R., Bonté F., Dumas M., Verbavatz J. M. (2002). Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J. Invest. Dermatol. 118, 678–685 10.1046/j.1523-1747.2002.01710.x [DOI] [PubMed] [Google Scholar]
- Sugiyama Y., Ota Y., Hara M., Inoue S. (2001). Osmotic stress up-regulates aquaporin-3 gene expression in cultured human keratinocytes. Biochim. Biophys. Acta 1522, 82–88 10.1016/S0167-4781(01)00320-7 [DOI] [PubMed] [Google Scholar]
- Tattersall D., Scott C. A., Gray C., Zicha D., Kelsell D. P. (2009). EKV mutant connexin 31 associated cell death is mediated by ER stress. Hum. Mol. Genet. 18, 4734–4745 10.1093/hmg/ddp436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth D. M., Szoke E., Bölcskei K., Kvell K., Bender B., Bosze Z., Szolcsányi J., Sándor Z. (2011). Nociception, neurogenic inflammation and thermoregulation in TRPV1 knockdown transgenic mice. Cell. Mol. Life Sci. 68, 2589–2601 10.1007/s00018-010-0569-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. L., Chang W., Bikle D. D. (2005). Phospholipase cgamma1 is required for activation of store-operated channels in human keratinocytes. J. Invest. Dermatol. 124, 187–197 10.1111/j.0022-202X.2004.23544.x [DOI] [PubMed] [Google Scholar]
- Voss K. E., Bollag R. J., Fussell N., By C., Sheehan D. J., Bollag W. B. (2011). Abnormal aquaporin-3 protein expression in hyperproliferative skin disorders. Arch. Dermatol. Res. 303, 591–600 10.1007/s00403-011-1136-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiszniewski L., Limat A., Saurat J. H., Meda P., Salomon D. (2000). Differential expression of connexins during stratification of human keratinocytes. J. Invest. Dermatol. 115, 278–285 10.1046/j.1523-1747.2000.00043.x [DOI] [PubMed] [Google Scholar]
- Xiao R., Tian J., Tang J., Zhu M. X. (2008). The TRPV3 mutation associated with the hairless phenotype in rodents is constitutively active. Cell Calcium 43, 334–343 10.1016/j.ceca.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Y., Charles R. P., Hummler E., Baines D. L., Isseroff R. R. (2013). The epithelial sodium channel mediates the directionality of galvanotaxis in human keratinocytes. J. Cell Sci. 126, 1942–1951 10.1242/jcs.113225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J. W., Seo J. A., Jeong Y. S., Bae I. H., Jang W. H., Lee J., Kim S. Y., Shin S. S., Woo B. Y., Lee K. W. et al. (2011). TRPV1 antagonist can suppress the atopic dermatitis-like symptoms by accelerating skin barrier recovery. J. Dermatol. Sci. 62, 8–15 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.