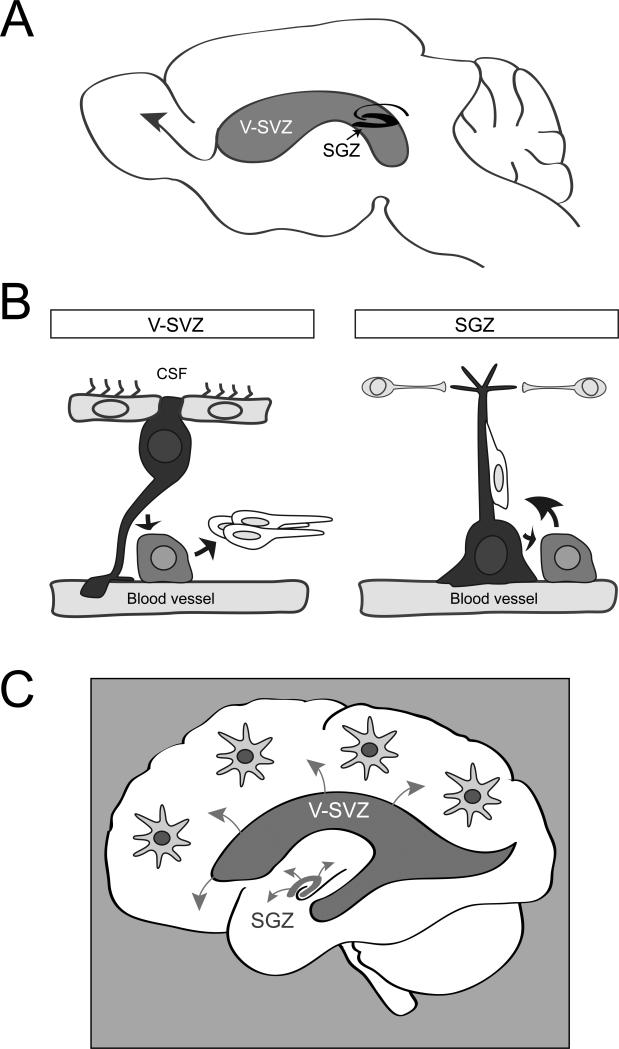
Figure 2. Endogenous adult neural stem cells and their niche.
A. Schema of a sagittal section of an adult mouse brain showing the V-SVZ adjacent to the lateral ventricles and SGZ of the hippocampal formation. New neurons born in the V-SVZ migrate a long distance to their final destination in the olfactory bulb (arrow). In contrast, adult-born neurons in the SGZ integrate locally into the circuitry.
B Schema of cell types and the anatomy of adult neurogenic niches. The V-SVZ niche (left) is composed of astrocyte NSCs (black) which contact the ventricular lumen between multiciliated ependymal cells, and which extend a radial process that contacts blood vessels. These cells give rise to amplifying progenitors (light grey), which in turn differentiate to migrating immature neurons (white). These immature neurons migrate to the olfactory bulbs, where they mature into interneurons. NSCs and progenitors often directly contact blood vessels at specialized sites that lack astrocyte end feet. The SGZ niche (right) is also composed of astrocyte NSCs (black) in contact with blood vessels, but these cells do not contact the ventricular lumen. SGZ NSCs also give rise to immature neurons through progenitors, and these neurons travel along the radial processes of the NSCs to integrate within the local circuitry. Local interneurons regulate SGZ NSCs.
C Schema showing adult neural stem cell niches in the human brain. Future mechanistic studies on the biology of both regions will provide important insight into how to harness these endogenous NSCs and exploit their therapeutic potential. By modulating molecular pathways that regulate adult NSCs, it may eventually be possible to stimulate astrocytes elsewhere in the brain to become NSCs.