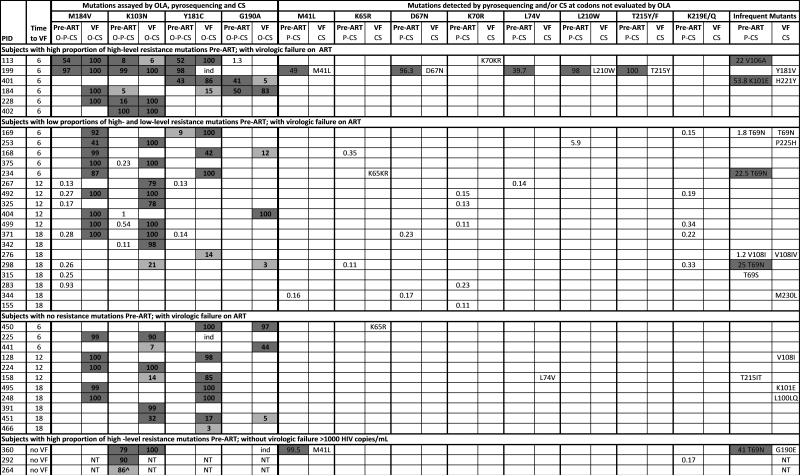
Figure 3.
Drug resistance mutations detected by OLA, pyrosequencing and consensus sequencing at baseline, and by OLA and consensus sequencing at virologic failure.
Note: Pre-ART specimens were tested by OLA (O), pyrosequencing (P), and consensus sequencing (CS); virologic failure (VF) specimens were tested by OLA and CS only. Numbers indicate the % mutant virus quantified by OLA (bold font, limit of detection = 2%) or pyrosequencing (regular font, limit of detection 0.1%). Dark grey indicates mutation detected by all methods evaluated. Light grey indicates mutation not detected by consensus sequencing, except as indicated for subject #264. Numbers with no shading indicate mutations detected by pyrosequencing only. NT = not tested (due to undetectable HIV viral load). ind = indeterminate result by OLA due to the presence of different mutation. ^ = mutation detected by OLA and consensus sequencing but not by pyrosequencing due to sequence polymorphisms in the region of the pyrosequencing PCR primers.