Abstract
Solid organ transplant recipients have elevated cancer risks, due in part to pharmacologic immunosuppression. However, little is known about risks for hematologic malignancies of myeloid origin. We linked the US Scientific Registry of Transplant Recipients with 15 population-based cancer registries to ascertain cancer occurrence among 207,859 solid organ transplants (1987–2009). Solid organ transplant recipients had significantly elevated risk for myeloid neoplasms, with standardized incidence ratios (SIRs) of 4.6 (95% confidence interval 3.8–5.6; N=101) for myelodysplastic syndromes (MDS), 2.7 (2.2–3.2; N=125) for acute myeloid leukemia (AML), 2.3 (1.6–3.2; N=36) for chronic myeloid leukemia, and 7.2 (5.4–9.3; N=57) for polycythemia vera. SIRs were highest among younger individuals and varied by time since transplantation and organ type (Poisson regression P<0.05 for all comparisons). Azathioprine for initial maintenance immunosuppression increased risk for MDS (P=0.0002) and AML (2–5 years after transplantation, P=0.0163). Overall survival following AML/MDS among transplant recipients was inferior to that of similar patients reported to US cancer registries (log-rank P<0.0001). Our novel finding of increased risks for specific myeloid neoplasms after solid organ transplantation supports a role for immune dysfunction in myeloid neoplasm etiology. The increased risks and inferior survival should heighten clinician awareness of myeloid neoplasms during follow-up of transplant recipients.
INTRODUCTION
In the United States (US), nearly 30 000 patients annually undergo solid organ transplantation.1 Clinical advances have led to substantial improvements in survival following transplantation, increasing the public health and clinical importance of understanding the long-term health effects of solid organ transplantation. The elevated cancer risks experienced by transplant recipients, largely due to pharmacologic immunosuppression to prevent graft rejection, are a key cause of morbidity and mortality following transplantation.1–3
Post-transplantation lymphoproliferative disorders (PTLDs) are among the most common serious complications of transplantation,1 but much less is known about the risks for hematologic malignancies of myeloid origin. Increased risks have been reported after solid organ transplantation for all myeloid neoplasms combined4, 5 and for acute myeloid leukemia (AML).6, 7 However, myeloid neoplasms comprise a range of diseases – including AML, myelodysplastic syndrome (MDS, which may progress to AML), chronic myeloid leukemia (CML), and other rarer entities such as polycythemia vera.8 Survival following a myeloid neoplasm diagnosis is generally poor, with estimated 5-year relative survival of 22% for AML, 41% for MDS, and 68% for CML in the US.9 Exposure to ionizing radiation and cytotoxic chemotherapy are established risk factors for certain myeloid neoplasms,10 but otherwise the causes of these malignancies remain unclear.8, 11 Evidence increasingly supports a role for immune dysfunction in the development of myeloid neoplasms, with elevated risks observed for individuals with a history of certain infections and autoimmune disease12–15 or HIV/AIDS.16, 17
We therefore conducted the first comprehensive investigation of the spectrum of risks for specific myeloid neoplasms among 207 859 solid organ transplants occurring in the US during 1987–2009 in the Transplant Cancer Match Study.2
METHODS
Transplant Cancer Match Study
The Transplant Cancer Match Study (www.transplantmatch.cancer.gov)2 provides comprehensive, systematic cancer ascertainment for solid organ transplant recipients by linking data from the Scientific Registry of Transplant Recipients (SRTR) with population-based cancer registries. The SRTR includes detailed information on all US solid organ transplants since 1987. Structured data are obtained regularly from transplant centers, including information on recipients (e.g., demographics, medical history, indication for transplant [Supplemental Table]), type of organ transplanted, and medications used for induction and baseline maintenance of immunosuppression to prevent graft rejection.
During 2008–2012, serial record linkages were completed between the SRTR and 15 population-based cancer registries: California (years of coverage: 1988–2008), Colorado (1988–2009), Connecticut (1973–2009), Florida (1981–2009), Georgia (1995–2008), Hawaii (1973–2007), Illinois (1986–2007), Iowa (1973–2009), Michigan (1985–2009), North Carolina (1990–2007), New Jersey (1979–2006), New York (1976–2007), Seattle/Puget-Washington (1974–2008), Texas (1995–2006), and Utah (1973–2008). Transplant recipients residing in these registry areas during the specified time periods were eligible for this analysis (46% of the US transplant population). We further excluded transplant recipients with unknown race/ethnicity (N=1421) or history of HIV (N=238). The study was approved by human subjects research review committees at the National Cancer Institute and, as required, participating cancer registries.
Myeloid Neoplasm Ascertainment
Incident diagnoses of myeloid neoplasms were identified through the cancer registries using International Classification of Diseases for Oncology, 3rd Edition morphology codes,18 grouped according to the World Health Organization8 classification into AML (9840, 9861, 9866–9867, 9870–9874, 9891, 9895–9897, 9910, 9920, 9930–9931), MDS (9980–9989), CML (9863, 9875–9876), chronic myelomonocytic leukemia (CMML; 9945–9946), polycythemia vera (9950), and other chronic myeloproliferative disorders (9960–9964, 9975; combined due to small numbers of observed cases). CMML, MDS, polycythemia vera, and other chronic myeloproliferative disorders only became reportable to cancer registries in 2001 and, thus, were not ascertained prior to 2001.
Statistical Analysis
For each transplant, follow-up began on the date of transplantation, start of cancer registry coverage, or (for evaluation of CMML, MDS, polycythemia vera, and other chronic myeloproliferative diseases) January 1, 2001, whichever came last. Follow-up ended at the earliest of: myeloid neoplasm diagnosis, graft failure, re-transplantation, death, loss to follow-up, or end of cancer registry coverage. Follow-up time ended at graft failure or re-transplantation due to substantial changes in clinical status (e.g., immunosuppression medication use) associated with these events.
To quantify risk of myeloid neoplasms after solid organ transplantation in comparison with the general population, we used standardized incidence ratios (SIRs). Expected numbers of cases were calculated by applying myeloid neoplasm incidence in the cancer registry areas to the person-time at risk of the transplant recipients, stratifying on age (5-year groups), sex, race/ethnicity, calendar year, and registry. Exact 95% confidence intervals (CIs) about the SIR were computed based on the Poisson distribution. We compared myeloid neoplasm SIRs for recipient subgroups (e.g., defined by age at transplantation or organ type) using a Wald test (Phomogeneity) derived from multivariate Poisson regression models that included terms for age at transplantation, sex, race, transplanted organ, and year of transplantation.
We conducted a sensitivity analysis restricted to those transplants performed during years when cancer registries captured the cancers of interest (i.e., transplants for which follow-up began at the date of transplantation). For AML and CML, this analysis included transplants occurring after the start of cancer registry coverage (N=202 626 transplants). For CMML, MDS, polycythemia vera, and other chronic myeloproliferative disorders, this analysis included transplants occurring in 2001 or later (N=105 130 transplants). Because the occurrence of a previous cancer may alter risk of subsequent myeloid neoplasms, we conducted a second sensitivity analysis restricting the cohort to individuals with no history of cancer prior to transplantation and censoring people when they developed any cancer (for AML and CML: N=194 355 transplants; for CMML, MDS, polycythemia vera, and other chronic myeloproliferative disorders: N=105 130). Results from these sensitivity analyses were similar to the main results and, thus, are not presented.
To understand the clinical impact of myeloid neoplasms in solid organ transplant recipients, we conducted two types of analyses. First, we estimated the relative risk (RR) of mortality from any cause associated with myeloid neoplasm development using hazard ratios (HRs) and 95% CIs derived from multivariate Cox regression models. Models used time since transplantation as the time scale and were adjusted for age at transplantation, sex, race, transplanted organ, and year of transplantation, with a time-dependent covariate indicating myeloid neoplasm diagnosis. Second, we constructed Kaplan-Meier curves for overall survival (i.e., time before death due to any cause) after myeloid neoplasm diagnosis, comparing transplant recipients to individuals with the same diagnoses reported to 17 SEER cancer registries.9 Because demographic factors are important determinants of overall survival, we individually-matched 10 SEER cases (selected randomly) to each case from the transplant population by age and calendar year of myeloid neoplasm diagnosis (±5 years), sex, and race/ethnicity. Overall survival was then compared between the two patient populations using the log-rank test.
RESULTS
Among 207 859 solid organ transplants occurring during 1987–2009 (950 464 person-years of follow-up), kidney was the most frequently transplanted organ (58%), followed by liver (22%), heart (10%), lung (4%), and other or multiple organs (6%, Table 1). The majority of recipients were male (61%) and non-Hispanic white (62%), and most transplants occurred at ages 35–64 years (67%). Recipient characteristics varied by type of transplanted organ. In particular, the proportion of males ranged from 52% for lung recipients to 75% for heart recipients, and the proportion of individuals aged ≥50 years at transplantation ranged from 21% for recipients of other/multiple organs to 59% for lung recipients.
Table 1.
Recipient characteristics for 207 859 solid organ transplants* in the United States, 1987–2009
| Type of organ transplanted | |||||||
|---|---|---|---|---|---|---|---|
| Total (N=207 859) |
Kidney (N=120 307) |
Liver (N=45 742) |
Heart (N=20 758) |
Lung (N=8 543) |
Other/ multiple** (N=12 509) |
||
| Characteristic | N | % | % | % | % | % | % |
| Sex | |||||||
| Male | 126 944 | 61 | 60 | 61 | 75 | 52 | 58 |
| Female | 80 915 | 39 | 40 | 39 | 25 | 48 | 42 |
| Age at Transplantation, years | |||||||
| 0–19 | 18 356 | 9 | 7 | 12 | 15 | 6 | 6 |
| 20–34 | 31 621 | 15 | 19 | 6 | 8 | 13 | 24 |
| 35–49 | 64 871 | 31 | 33 | 29 | 20 | 21 | 50 |
| 50–64 | 75 430 | 36 | 32 | 44 | 48 | 51 | 19 |
| 65+ | 17 581 | 8 | 9 | 8 | 9 | 8 | 2 |
| Race/Ethnicity | |||||||
| White, Non–Hispanic | 129 379 | 62 | 54 | 70 | 74 | 86 | 76 |
| Black, Non–Hispanic | 35 185 | 17 | 22 | 9 | 14 | 7 | 11 |
| Hispanic | 31 902 | 15 | 17 | 16 | 9 | 6 | 11 |
| Asian/Pacific Islander | 11 393 | 5 | 7 | 5 | 3 | 1 | 2 |
| Transplant Number | |||||||
| First | 189 463 | 91 | 91 | 91 | 97 | 96 | 82 |
| Second | 16 816 | 8 | 9 | 8 | 3 | 4 | 16 |
| Third or Higher | 1 580 | 1 | 1 | 1 | 0 | 0 | 2 |
| Calendar Year of Transplantation | |||||||
| 1987–1994 | 38 506 | 19 | 19 | 18 | 26 | 13 | 13 |
| 1995–1999 | 52 361 | 25 | 25 | 25 | 28 | 25 | 26 |
| 2000–2004 | 64 715 | 31 | 31 | 32 | 28 | 31 | 34 |
| 2005–2009 | 52 277 | 25 | 25 | 26 | 19 | 32 | 27 |
Includes 207,859 transplants occurring among 192 562 individuals.
Other/multiple includes kidney-pancreas (N=7267), pancreas (N=1892), kidney-liver (N=1743), heart-lung (N=427), kidney-heart (N=287), intestine (N=279), liver-intestine (N=229), pancreas-liver-intestine (N=181), and other rare solid organ transplants (N=204).
Compared with the general population, solid organ transplant recipients had significantly and substantially elevated risks for myeloid neoplasms, with risks (SIRs) increased 2.7-fold (95%CI 2.2–3.2) for AML, 4.6-fold (3.8–5.6) for MDS, 2.3-fold (1.6–3.2) for CML, and 7.2-fold (5.4–9.3) for polycythemia vera (Table 2). Risks were non-significantly elevated 2.1-fold (0.8–4.6) for CMML (N=6) and 1.8-fold (1.0–2.9) for other chronic myeloproliferative disorders (N=16), but the small numbers of observed cases for these entities precluded further analysis.
Table 2.
Risk of specific myeloid neoplasms overall and by type of organ transplanted among 207 859 solid organ transplants in the United States, 1987–2009
| Acute myeloid leukemia | Myelodysplastic syndromes | Chronic myeloid leukemia | Polycythemia vera | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | SIR | 95% CI | N | SIR | 95% CI | N | SIR | 95% CI | N | SIR | 95% CI |
| Total | 125 | 2.7 | 2.2–3.2 | 101 | 4.6 | 3.8–5.6 | 36 | 2.3 | 1.6–3.2 | 57 | 7.2 | 5.4–9.3 |
| Transplanted Organ | ||||||||||||
| Kidney | 46 | 1.8 | 1.3–2.5 | 45 | 3.8 | 2.8–5.1 | 20 | 2.3 | 1.4–3.6 | 37 | 9.0 | 6.4–12.4 |
| Liver | 38 | 3.5 | 2.5–4.8 | 22 | 4.4 | 2.7–6.6 | 8 | 2.4 | 1.0–4.7 | 11 | 5.7 | 2.8–10.2 |
| Heart | 29 | 3.7 | 2.5–5.3 | 17 | 4.5 | 2.6–7.2 | 5 | 2.1 | 0.7–4.9 | 3 | 2.3 | 0.5–6.6 |
| Lung | 10 | 6.5 | 3.1–11.9 | 11 | 14.3 | 7.2–25.7 | <3 | 2.1 | 0.1–11.6 | 0 | 0.0 | 0.0–12.2 |
| Other/multiple | <3 | 1.4 | 0.2–5.1 | 6 | 14.0 | 5.2–30.5 | <3 | 3.4 | 0.4–12.3 | 6 | 21.7 | 8.0–47.2 |
| Phomogeneity* | 0.0126 | 0.0001 | 0.9997 | 0.1580 | ||||||||
Exact cell counts with <3 cases are not presented to protect patient confidentiality.
P from a Wald chi-square test of homogeneity derived from a multivariate Poisson regression model adjusted for sex, age at transplantation, race, and calendar year of transplantation.
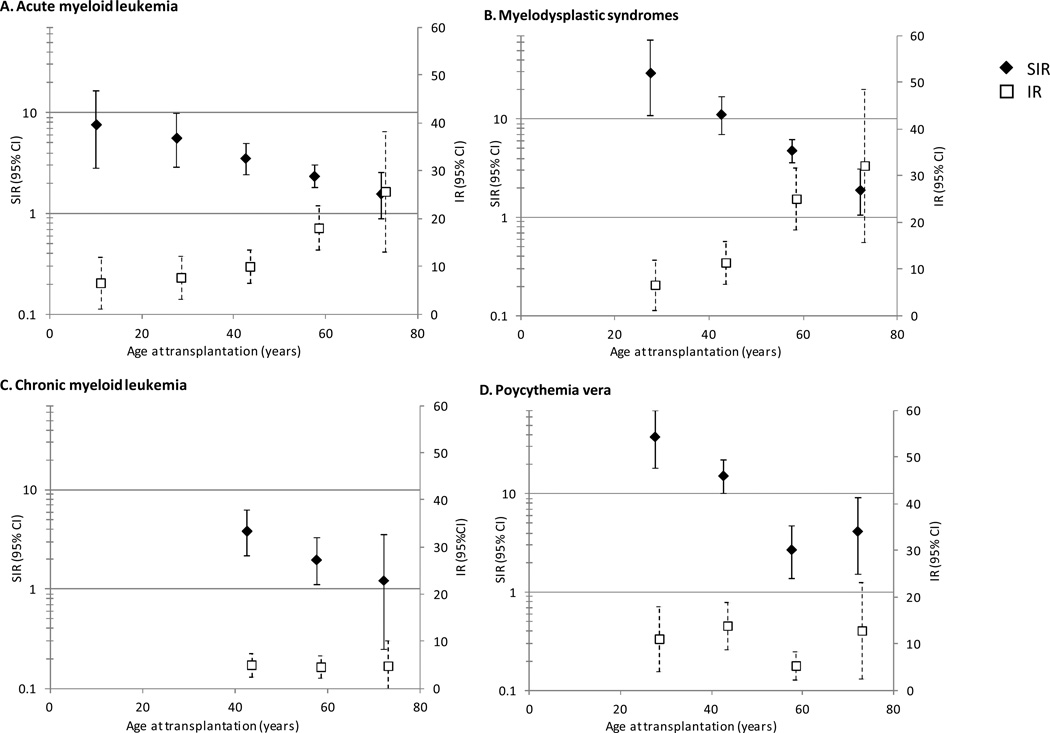
Incidence of AML and MDS increased with increasing age at transplantation, reaching 32.2 and 25.7 cases/100 000 person-years, respectively, for individuals aged ≥65 years at transplantation (Figure 1). In contrast, incidence of CML was approximately 5 cases/100 000 person-years for ages 35–49, 50–64, and ≥65 years, whereas incidence of polycythemia vera was approximately 12 cases/100 000 person-years for ages 20–34, 35–49, and ≥65 years but only 5 cases/100 000 person-years for ages 50–64. Unlike the incidence patterns, SIRs for all myeloid neoplasms (reflecting risk relative to the general population) were highest in young recipients and decreased monotonically with increasing age, with the exception of polycythemia vera.
Figure 1. Incidence rates (per 100 000 person-years) and standardized incidence ratios* for specific myeloid neoplasms by age at transplantation among 207 859 solid organ transplants in the United States, 1987–2009.
Abbreviations: confidence interval (CI), incidence rate (IR), standardized incidence ratio (SIR). SIRs are presented for 0–19, 20–34, 35–49, 50–64, and 65+ years, with estimates centered over these intervals.
* IRs and SIRs are not presented when <3 cases were observed due to imprecise estimates.
For AML and polycythemia vera, SIRs also varied significantly by time since transplantation (Figure 2). Risks for AML were increased 2.0-fold during the first year following transplantation and peaked at 4.6-fold during 1–1.9 years following transplantation, with declining risk thereafter (Phomogeneity=0.0456). In contrast, polycythemia vera risk was increased 22.0-fold during the first year following transplantation, with SIRs dropping precipitously thereafter, yet remaining significantly increased 2.9–6.7-fold (Phomogeneity=0.0003). For MDS, risks appeared to decline consistently during the first five years following transplantation and increase again thereafter, although these changes were not statistically significant (Phomogeneity=0.5170). For CML, risk did not vary by time since transplantation (Phomogeneity=0.8049).
Figure 2. Standardized incidence ratios* for specific myeloid neoplasms by time since transplantation among 207 859 solid organ transplants in the United States, 1987–2009.
Abbreviations: confidence interval (CI), standardized incidence ratio (SIR). SIRs are presented for <1, 1–1.9, 2–2.9, 3–3.9, 4–4.9, 5–6.9, 7–8.9, and 9+ years, with estimates centered over these intervals.
* SIRs are not presented when <3 cases were observed due to imprecise estimates.
In analyses by type of organ transplanted, risk patterns differed for specific myeloid neoplasms (Table 2). For MDS, risks were strikingly high among lung recipients (SIR=14.3) and recipients of other/multiple organs (SIR=14.0) but substantially lower, albeit still significantly elevated compared with the general population, among heart (SIR=4.5), liver (SIR=4.4) and kidney (SIR=3.8) recipients (Phomogeneity=0.0001). Risks for AML also were highest among lung recipients (SIR=6.5) but were lowest for recipients of other/multiple organs (SIR=1.4; Phomogeneity=0.0126). In contrast, risks for polycythemia vera were 9.0-fold increased among kidney recipients and 21.7-fold increased among recipients of other/multiple organs, although these differences in risk by organ type were not significant (Phomogeneity=0.1580); the strikingly elevated risk for polycythemia vera in the first year following transplantation also was evident across organ type (data not shown). Risks for CML did not differ significantly by organ type (Phomogeneity=0.9997).
To further understand differences in myeloid neoplasm risk by the type of organ transplanted, we explored risks according to receipt of immunosuppressive medications to prevent graft rejection and indication for transplantation. Individuals who received azathioprine for initial maintenance of immunosuppression had significantly higher risk for MDS (SIR=8.4 vs. 3.5, P=0.0002) and for AML occurring 2–5 years after transplantation (SIR=6.6 vs. 1.7, P=0.0163; with no association for AML occurring <2 or >5 years after transplantation), whereas no association was observed for CML or polycythemia vera. Inclusion of azathioprine in the multivariate Poisson regression models for AML and MDS reduced the heterogeneity in risks observed by organ type. No other therapies for induction of immunosuppression at the time of transplantation (monoclonal antibodies, polyclonal antibodies, alemtuzumab, or interleukin-2 receptor antagonists) or for initial maintenance of immunosuppression (cyclosporine, tacrolimus, mycophenolate mofetil, mTOR inhibitors, steroids) were associated with myeloid neoplasm risk (data not shown). We also did not observe significantly increased risks of myeloid neoplasms associated with broadly defined indications for transplantation (data not shown).
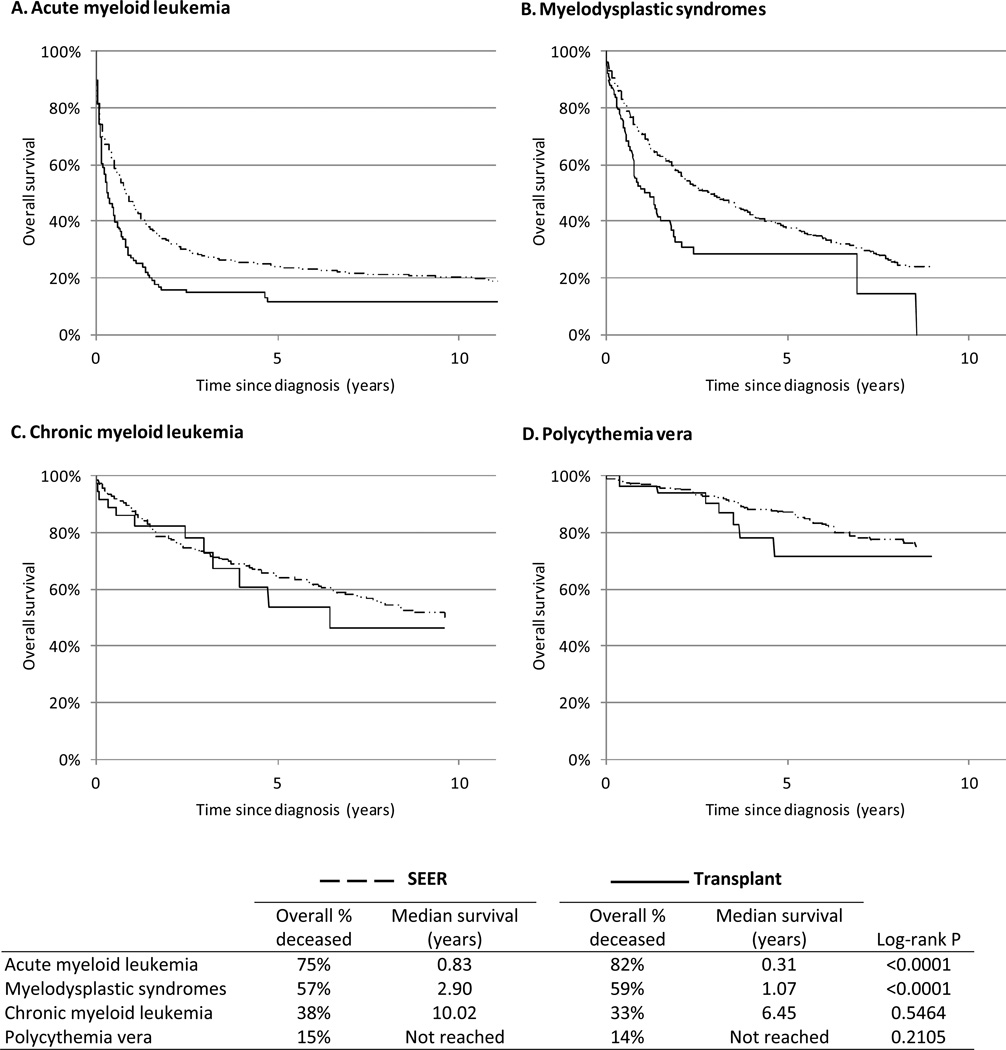
Within the cohort of transplant recipients, risk of death was significantly increased 14.4-fold (95%CI 11.8–17.6) following the diagnosis of AML, 6.8-fold (5.2–8.7) following MDS, and 2.4-fold (1.3–4.4) following CML, but not significantly increased following the diagnosis of polycythemia vera (RR=1.2, 95%CI 0.6–2.4). Compared to patients reported to SEER cancer registries, overall survival was consistently inferior among transplant patients with AML (median survival: transplant patients=0.31 years, SEER patients=0.83 years; log-rank P<0.0001) and MDS (median survival: transplant patients=1.07 years, SEER patients=2.90 years; P<0.0001) (Figure 3). In contrast, overall survival following CML and polycythemia vera was lower, but not significantly, in transplant patients compared to patients reported to SEER cancer registries (P=0.5464 and 0.2105, respectively).
Figure 3. Overall survival following myeloid neoplasm diagnosis among 207 859 solid organ transplants in the United States, 1987–2009, compared with matched* patients reported to the SEER Program.
Abbreviations: Surveillance, Epidemiology, and End Results (SEER), Scientific Registry of Transplant Recipients (SRTR).
* A sample of cases of each myeloid neoplasm type was selected from SEER, individually-matching 10 SEER cases per transplant case by age and calendar year of myeloid neoplasm diagnosis (±5 years), sex, and race.
DISCUSSION
By combining national data on solid organ transplantation with systematic cancer ascertainment from population-based registries, we provide the first comprehensive study demonstrating that transplant recipients have significantly elevated risk for a range of myeloid neoplasms. Although these malignancies are relatively rare, the increased risks as well as the inferior survival following diagnosis, particularly for AML and MDS, should heighten the clinical awareness of myeloid neoplasms during long-term follow-up of solid organ transplant recipients.
Our results support a role for immune dysfunction in myeloid neoplasm etiology, consistent with the observations of increased myeloid neoplasm risk among individuals with HIV/AIDS16, 17 or a history of autoimmune disease and infections.12–15 The persistently elevated risks for myeloid neoplasms following transplantation varied by type of organ transplanted and time since transplantation, with no clear association with specific indications for transplantation. Our findings contrast with the U-shaped pattern of risk by time since transplantation for NHL,19, 20 for which early-onset disease risks are attributed to Epstein-Barr virus infection and receipt of T-cell depleting polyclonal antibodies, and later-onset disease risks are attributed to longer-term immunosuppression.19, 21, 22
AML and MDS were the most commonly occurring myeloid neoplasms in solid organ transplant recipients. The higher risks we observed for lung recipients compared with recipients of other organs were attributable in part to the increased use of azathioprine, which was associated with increased risk of AML and MDS. Our findings are consistent with a previous study of AML among solid organ transplant recipients6 and confirm previous reports of increased AML/MDS risk associated with use of azathioprine or other antimetabolites.6, 23–28 In current clinical practice in the US, azathioprine is used infrequently in solid organ transplant recipients, having been largely replaced by mycophenolate mofetil.29 Although the lack of association between myeloid neoplasm risk and mycophenolate mofetil or any of the other immunosuppressive drugs we evaluated is reassuring, our results should be interpreted cautiously in light of the potential for incomplete ascertainment of medication use, lack of information on long-term use, and our inability to evaluate drug doses.30 Clinical awareness of myeloid neoplasm risk after solid organ transplantation is particularly relevant for MDS/AML due to the improved outcomes for patients treated for MDS before progression to AML.
We also observed elevated risks for CML and polycythemia vera. CML is a relatively rare malignancy with a largely unknown etiology. The elevated risks observed in our study were consistent with those reported previously in the Transplant Cancer Match study,2 as well as another study.5 Albeit based on small numbers of cases, we did not observe significant variation in CML risk by recipient subgroup, unlike the other myeloid neoplasms. Our finding of elevated risks for polycythemia vera among solid organ transplant recipients, particularly in the first year following transplantation, has not been reported in the literature. This association may be spurious, particularly the strikingly elevated risks in the first year following transplantation, for example resulting from misdiagnosis among kidney recipients with a history of erythropoietin use31 or incidental diagnosis of previously undetected disease, particularly in liver recipients with Budd-Chiari syndrome.32 However, risks of polycythemia vera remained significantly elevated for the duration of post-transplantation follow-up in this study, supporting a causal role for transplantation in polycythemia vera development.
Further research is warranted to explore the biological mechanisms that may contribute to the development of myeloid neoplasms following solid organ transplantation. Pharmacologic immunosuppression to prevent graft rejection may result in loss of critical immunosurveillance function, as described for CML.33 Alternatively, a role for immune stimulation in AML etiology is supported by a recent report of elevated serum kappa and lambda immunoglobulin free light chains (FLCs) prior to the development of AML.34 That observation is particularly intriguing in light of similar observations for lymphoma risk after solid organ transplantation35 or HIV/AIDS.36 Further research also is warranted to clarify the importance of direct cytotoxicity versus immunosuppression intensity or duration of certain immunosuppressive agents in myeloid neoplasm etiology.
The incidence of most myeloid neoplasms increased with increasing age. However, the SIR for each specific myeloid neoplasm type was significantly higher among younger versus older individuals at transplantation, and the median age at diagnosis among transplant recipients compared with SEER population-based cases ranged from 17 years younger for MDS to 5 years younger for CML. This risk pattern may result from intrinsic susceptibility of young individuals to the direct or indirect carcinogenic effects of immunosuppressive medications. Alternatively, transplantation may have a larger relative effect in younger individuals, who have had less time to accumulate carcinogenic exposures that may lead to myeloid neoplasm development.
We present the first large-scale analysis demonstrating that overall survival in transplant recipients was significantly inferior for AML and MDS compared with patients diagnosed with these malignancies reported to SEER cancer registries. Optimal treatment approaches for myeloid neoplasms following transplantation are not known, although normal kidney and liver function are required for a number of standard chemotherapeutic regimens. Unfortunately, data on myeloid neoplasm treatments, cytogenetic abnormalities, and comorbidities were not available for transplant recipients, and, thus, we could not determine whether the observed differences in survival may be explained by differences in these factors. Additionally, we lacked detailed data on cause of death, and some deaths in the transplant cohort may have been due to transplant-related disease.
The linkage of two major population-based data sources enabled us to conduct the first assessment of risk for the spectrum of myeloid neoplasms following solid organ transplantation. The population-based nature of the data eliminated the biases associated with previous clinical series, whereas the large sample size facilitated investigation of less common myeloid neoplasms as well as estimation of risks among patient subgroups. However, several limitations of our study should be considered in the interpretation of our results. Because cancer diagnoses were ascertained through central registries, standardized pathology review was not feasible. Although diagnostic criteria for specific myeloid neoplasms has changed over time,8 the impact of these changes on our results was minimized by comparing myeloid neoplasm incidence in solid organ transplant recipients to that in the general population, which was presumably affected by similar changes. Risks may have been underestimated because cancers diagnosed in patients who had migrated out of the cancer registry areas were not ascertained, although analyses of migration of the transplant population suggest that this affects only a small number of individuals.2 Finally, despite the large sample size, our statistical power for evaluating risks in certain patient subgroups remained limited.
In summary, we observed significantly elevated risks for AML, MDS, CML, and polycythemia vera after solid organ transplantation. Diagnoses of AML, MDS, and CML were associated with an increased risk of death within the transplant cohort, and overall survival following AML and MDS in transplant recipients was significantly inferior when compared with patients with the same diagnoses reported to SEER cancer registries. Our findings call for an increased awareness of myeloid neoplasms in the risk/benefit assessment for solid organ transplantation and during long-term follow-up of solid organ transplant recipients. Additionally, further research is needed to elucidate the role of immune dysfunction in myeloid neoplasm etiology and to identify optimal treatments for myeloid neoplasms in immunosuppressed individuals.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported in part by the Intramural Program of the National Cancer Institute, National Institutes of Health, and the Surveillance, Epidemiology, and End Results Program. The authors gratefully acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration (Monica Lin), the SRTR (Ajay Israni, Bertram Kasiske, Paul Newkirk, Jon Snyder), and the following cancer registries: the states of California, Colorado (Jack Finch), Connecticut (Lou Gonsalves), Georgia (Rana Bayakly), Hawaii (Brenda Hernandez), Iowa, Illinois (Lori Koch), Michigan (Glenn Copeland), New Jersey (Xiaoling Niu), New York (Amy Kahn), North Carolina (Chandrika Rao), Texas (Melanie Williams), and Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington (Margaret Madeleine). We also thank analysts at Information Management Services for programming support (David Castenson, Matthew Chaloux, Michael Curry, Ruth Parsons).
The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, Health Resources and Services Administration, SRTR, cancer registries, or their contractors. This research was supported in part by the Intramural Research Program of the National Cancer Institute.
During the initial period when registry linkages were performed, the SRTR was managed by Arbor Research Collaborative for Health in Ann Arbor, MI (contract HHSH234200537009C); beginning in September 2010, the SRTR was managed by Minneapolis Medical Research Foundation in Minneapolis, MN (HHSH250201000018C). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP000817-05), Illinois (5658DP000805-04), Michigan (5U58DP000812-03), New Jersey (5U58/DP000808-05), New York (U58DP0038789), North Carolina (U58DP000832), and Texas (5U58DP000824-04). The following cancer registries were supported by the SEER Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201000024C), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (HSN261201000032C and N01-PC-35143), New Jersey (HHSN261201000027C and N01-PC-54405), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN261201000026C). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, New Jersey, New York (Cancer Surveillance Improvement Initiative 14–2491), Texas, and Washington, as well as the Fred Hutchinson Cancer Research Center in Seattle, WA.
Footnotes
Supplementary information is available at Leukemia's website.
AUTHORSHIP CONTRIBUTIONS
Designed research: LM Morton, TM Gibson, EA Engels
Collected data: CA Clarke, CF Lynch, EA Engels
Analyzed data: LM Morton, TM Gibson, EA Engels
Interpreted data and made critical contributions to manuscript: All authors.
Wrote the manuscript: LM Morton
CONFLICTS OF INTEREST
The authors have no competing financial interests to disclose.
REFERENCES
- 1.Annual Data Report of the US Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients (SRTR) Am J Transplant. 2013 Jan;13(Suppl 1):1–234. doi: 10.1111/ajt.12028. [DOI] [PubMed] [Google Scholar]
- 2.Engels EA, Pfeiffer RM, Fraumeni JF, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011 Nov 02;306(17):1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 4.Adami J, Gabel H, Lindelof B, Ekstrom K, Rydh B, Glimelius B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89(7):1221–1227. doi: 10.1038/sj.bjc.6601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinlan SC, Morton LM, Pfeiffer RM, Anderson LA, Landgren O, Warren JL, et al. Increased risk for lymphoid and myeloid neoplasms in elderly solid-organ transplant recipients. Cancer Epidemiol Biomarkers Prev. 2010 Jun;19(5):1229–1237. doi: 10.1158/1055-9965.EPI-09-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Offman J, Opelz G, Doehler B, Cummins D, Halil O, Banner NR, et al. Defective DNA mismatch repair in acute myeloid leukemia/myelodysplastic syndrome after organ transplantation. Blood. 2004 Aug 01;104(3):822–828. doi: 10.1182/blood-2003-11-3938. [DOI] [PubMed] [Google Scholar]
- 7.Gale RP, Opelz G. Commentary: does immune suppression increase risk of developing acute myeloid leukemia? Leukemia. 2012 Aug 26;26:422–423. doi: 10.1038/leu.2011.224. [DOI] [PubMed] [Google Scholar]
- 8.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 9.Howlader N, Noone A, Krapcho M, Garshell J, Neyman N, Altekruse S, et al. SEER Cancer Statistics Review, 1975–2010, National Cancer Institute. Bethesda, MD: 2013. [Google Scholar]
- 10.Leone G, Fianchi L, Pagano L, Voso MT. Incidence and susceptibility to therapy-related myeloid neoplasms. Chem Biol Interact. 2010;184(1–2):39–45. doi: 10.1016/j.cbi.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Anderson LA, Duncombe AS, Hughes M, Mills ME, Wilson JC, McMullin MF. Environmental, lifestyle, and familial/ethnic factors associated with myeloproliferative neoplasms. Am J Hematol. 2012 Feb;87(2):175–182. doi: 10.1002/ajh.22212. [DOI] [PubMed] [Google Scholar]
- 12.Anderson LA, Pfeiffer RM, Landgren O, Gadalla S, Berndt SI, Engels EA. Risks of myeloid malignancies in patients with autoimmune conditions. Br J Cancer. 2009 Mar 10;100(5):822–828. doi: 10.1038/sj.bjc.6604935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristinsson SY, Landgren O, Samuelsson J, Bjorkholm M, Goldin LR. Autoimmunity and the risk of myeloproliferative neoplasms. Haematologica. 2010 Jul;95(7):1216–1220. doi: 10.3324/haematol.2009.020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristinsson SY, Bjorkholm M, Hultcrantz M, Derolf AR, Landgren O, Goldin LR. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol. 2011 Jul 20;29(21):2897–2903. doi: 10.1200/JCO.2011.34.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramadan SM, Fouad TM, Summa V, Hasan S, Lo-Coco F. Acute myeloid leukemia developing in patients with autoimmune diseases. Haematologica. 2012 Jun;97(6):805–817. doi: 10.3324/haematol.2011.056283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frisch M, Biggar RJ, Engels EA, Goedert JJ for the A-CMRSG. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285(13):1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 17.Shiels MS, Engels EA. Increased risk of histologically defined cancer subtypes in human immunodeficiency virus-infected individuals: clues for possible immunosuppression-related or infectious etiology. Cancer. 2012 Oct 1;118(19):4869–4876. doi: 10.1002/cncr.27454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritz AG. International classification of diseases for oncology (ICD-O) 3rd edn. Geneva: World Health Organization; 2000. p. vii.p. 240. [Google Scholar]
- 19.van Leeuwen MT, Grulich AE, Webster AC, McCredie MRE, Stewart JH, McDonald SP, et al. Immunosuppression and other risk factors for early and late non-Hodgkin lymphoma after kidney transplantation. Blood. 2009 Jul 16;114(3):630–637. doi: 10.1182/blood-2009-02-202507. [DOI] [PubMed] [Google Scholar]
- 20.Clarke CA, Morton LM, Lynch C, Pfeiffer RM, Hall EC, Gibson TM, et al. Risk of lymphoma subtypes after solid organ transplantation in the United States. Br J Cancer. 2013 Jul 9;109(1):280–288. doi: 10.1038/bjc.2013.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinlan SC, Pfeiffer RM, Morton LM, Engels EA. Risk factors for early-onset and late-onset post-transplant lymphoproliferative disorder in kidney recipients in the United States. Am J Hematol. 2011 Mar;86(2):206–209. doi: 10.1002/ajh.21911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghobrial IM, Habermann TM, Macon WR, Ristow KM, Larson TS, Walker RC, et al. Differences between early and late posttransplant lymphoproliferative disorders in solid organ transplant patients: are they two different diseases? Transplantation. 2005;79(2):244–247. doi: 10.1097/01.tp.0000144335.39913.5c. [DOI] [PubMed] [Google Scholar]
- 23.Bo J, Schroder H, Kristinsson J, Madsen B, Szumlanski C, Weinshilboum R, et al. Possible carcinogenic effect of 6-mercaptopurine on bone marrow stem cells: relation to thiopurine metabolism. Cancer. 1999 Sep 15;86(6):1080–1086. doi: 10.1002/(sici)1097-0142(19990915)86:6<1080::aid-cncr26>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Relling MV, Yanishevski Y, Nemec J, Evans WE, Boyett JM, Behm FG, et al. Etoposide and antimetabolite pharmacology in patients who develop secondary acute myeloid leukemia. Leukemia. 1998 Apr;12(3):346–352. doi: 10.1038/sj.leu.2400928. [DOI] [PubMed] [Google Scholar]
- 25.Smith SM. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003 Apr 20;102(1):43–52. doi: 10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- 26.Kwong Y-L. Azathioprine: association with therapy-related myelodysplastic syndrome and acute myeloid leukemia. J Rheumatol. 2010 Mar;37(3):485–490. doi: 10.3899/jrheum.090834. 2010. [DOI] [PubMed] [Google Scholar]
- 27.Knipp S, Hildebrandt B, Richter J, Haas R, Germing U, Gattermann N. Secondary myelodysplastic syndromes following treatment with azathioprine are associated with aberrations of chromosome 7. Haematologica. 2005 Jun;90(5):691–693. [PubMed] [Google Scholar]
- 28.Putzki N, Knipp S, Ramczykowski T, Vago S, Germing U, Diener HC, et al. Secondary myelodysplastic syndrome following long-term treatment with azathioprine in patients with multiple sclerosis. Mult Scler. 2006 Jul;12(3):363–366. doi: 10.1191/135248506ms1307cr. [DOI] [PubMed] [Google Scholar]
- 29.Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer. 2008 Feb;8(1):24–36. doi: 10.1038/nrc2292. [DOI] [PubMed] [Google Scholar]
- 30.Dickinson DM, Bryant PC, Williams MC, Levine GN, Li S, Welch JC, et al. Transplant data: sources, collection, and caveats. Am J Transplant. 2004;4(Suppl 9):13–26. doi: 10.1111/j.1600-6135.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 31.Dutka P. Erythropoiesis-stimulating agents for the management of anemia of chronic kidney disease: past advancements and current innovations. Nephrol Nurs J. 2012 Nov-Dec;39(6):447–457. [PubMed] [Google Scholar]
- 32.Ulrich F, Pratschke J, Neumann U, Pascher A, Puhl G, Fellmer P, et al. Eighteen years of liver transplantation experience in patients with advanced Budd-Chiari syndrome. Liver Transpl. 2008 Feb;14(2):144–150. doi: 10.1002/lt.21282. [DOI] [PubMed] [Google Scholar]
- 33.Langabeer SE, Burke A, McCarron SL, Kelly J, Carroll P, Browne PV, et al. Chronic myeloid leukaemia presenting post-radiotherapy for prostate cancer: further evidence for an immunosurveillance effect. Br J Haematol. 2013 Sep;162(5):708–710. doi: 10.1111/bjh.12396. [DOI] [PubMed] [Google Scholar]
- 34.Wu SP, Costello R, Hofmann JN, Korde N, Mailankody S, Purdue M, et al. MGUS prevalence in a cohort of AML patients. Blood. 2013 Jul 11;122(2):294–295. doi: 10.1182/blood-2013-04-497859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engels EA, Preiksaitis J, Zingone A, Landgren O. Circulating antibody free light chains and risk of posttransplant lymphoproliferative disorder. Am J Transplant. 2012 May;12(5):1268–1274. doi: 10.1111/j.1600-6143.2011.03954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landgren O, Goedert JJ, Rabkin CS, Wilson WH, Dunleavy K, Kyle RA, et al. Circulating serum free light chains as predictive markers of AIDS-related lymphoma. J Clin Oncol. 2010 Feb 10;28(5):773–779. doi: 10.1200/JCO.2009.25.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.