SUMMARY
Stem cell population size is highly regulated across species and tissue types and alterations are associated with premature tissue failure or cancer. We assessed whether the tumor suppressor and mediator of cell contact inhibition Nf2/merlin plays a role in governing the hematopoietic stem cell pool by stem cell autonomous or niche determined processes. Hematopoietic stem cells in Nf2-deficient mice were increased in number and demonstrated a marked shift in location to the circulation. These changes were entirely dependent on changes in the microenvironment with a marked increase in trabecular bone and marrow vascularity associated with increased VEGF, but without cell autonomous alterations in stem cell characteristics. Nf2/merlin is critical for maintaining normal structure and function of the hematopoietic stem cell niche. It limits both bone and vascular components and our model suggests that it thereby constrains stem cell number and position.
INTRODUCTION
Stem cells reside in specialized microenvironments or ‘niches’ that are essential for their maintenance and proper function (Fuchs et al., 2004). Although components of hematopoietic stem cell (HSC) niches in the bone marrow have been defined, including osteoblasts, osteoclasts and possibly perivascular cells, questions remain about how heterologous cells contribute to HSC regulation both in terms of localization and function (Calvi et al., 2003; Kiel et al., 2005; Kollet et al., 2006; Zhang et al., 2003). A key role for the niche is to protect and maintain the stem cell pool by keeping the cells in a quiescent state and thereby preventing their exhaustion. However, the mobility of HSCs suggests that their interaction with the niche also is highly dynamic with a substantial tendency to disengage. The microenvironmental architecture of the HSC niche is complex with stem cells, stromal cells and vascular cells intermingled at the bone surface. It is likely that physical interactions and cell:cell communication between these different cellular components play important roles to keep the niche properly balanced and to maintain HSC homeostasis but the bases for these interactions are poorly defined.
The ubiquitously expressed tumor suppressor gene Neurofibromatosis 2 (Nf2) encodes a protein, merlin, which plays a key role in mediating cell:cell communication. Loss of merlin function is associated with development of multiple cancers in both humans and mice (McClatchey and Giovannini, 2005). Merlin is localized to the inner surface of the plasma membrane connecting membrane bound proteins to the actin cytoskeleton and thereby providing a link between external cues and the cell’s inner machinery (McClatchey and Giovannini, 2005). It has been shown that a characteristic of Nf2 deficiency in a range of primary cell types is a lack of contact-mediated inhibition of proliferation and an inability to form stable cadherin-mediated cell:cell junctions (Lallemand et al., 2003). A mechanism was recently demonstrated whereby merlin in osteoblasts coordinates stabilization of adherens junctions upon cell:cell contact and inhibits signaling through the epidermal growth factor receptor (EGFR) (Curto et al., 2007). Despite these profound effects in multiple cell types in vitro and its’ association with tumor development, little is known about the role of Nf2 in regulation of normal tissue homeostasis in vivo. A recent study showed that Nf2 is crucial for normal tissue morphogenesis during early embryonic development by regulating cell-cell adhesion during tissue fusion (McLaughlin et al., 2007).
Here we specifically addressed whether Nf2 plays a role in the regulation of stem cells within the hematopoietic compartment. We show that Nf2 deficient animals have dramatic HSC related phenotypes, affecting both localization (mobilization) and number (increased pool size) of the stem cells. However, these changes are not autonomous to the HSCs, rather they are bone marrow microenvironment-determined. Changes in the marrow include morphologic alterations with increased trabecular bone and vascularity associated with increased numbers of osteoblasts and VEGF respectively. Our findings therefore indicate that Nf2 plays a critical role in the maintenance of normal architecture in the bone marrow microenvironment and in so doing may provide a non-cell autonomous mechanism for modulating HSCs. Organization and content of the niche appears to dominate key parameters of stem cell homeostasis.
RESULTS
Inducible deletion of Nf2 in the mouse hematopoietic compartment
Nf2 is known to be expressed in most tissues and cell types. We examined the Nf2 mRNA levels in primitive subsets of hematopoietic cells and found robust expression in both the progenitor (lin−, c-kit+, Sca1−) and more immature (lin−, c-kit+, Sca1+) populations (Supplemental Fig. 1). To further establish a potential role for Nf2 in regulation of the hematopoietic compartment we used genetically engineered mice bearing conditional “floxed “ alleles of the Nf2 gene (Giovannini et al., 2000). We mated the floxed Nf2 mice with Mx1 Cre transgenic mice to generate an inducible knockout mouse model (Kuhn et al., 1995). Upon PolyIC administration Mx1Cre is known to efficiently induce gene deletion in all subsets of hematopoietic stem and progenitor cells (HSC/Ps) as well as in the bone marrow stroma (Larsson et al., 2003; Zhang et al., 2003). Accordingly, when we analyzed our mice 12 weeks after polyIC treatment, we found deletion of the Nf2 gene in 100% of hematopoietic colony-forming cells (CFCs) and a nearly complete deletion in adherent stroma cell layers derived from cultured bone marrow cells (Supplemental Fig. 2).
Both monoallelic and tissue specific biallelic Nf2 deletion in mice has been shown to trigger the formation of spontaneous tumors with age (Giovannini et al., 2000; McClatchey et al., 1998). As expected, we observed the occurrence of sporadic tumors in our mice from around 7 months after polyIC treatment and few animals survived beyond 8 months after knockout induction. Further investigations describing the nature of the tumors in these mice will be presented elsewhere. Here we focused our attention to the analysis of the hematopoietic compartment prior to malignant disease.
Marked shift in location to the circulation and increase in HSC number over time after Nf2 deletion
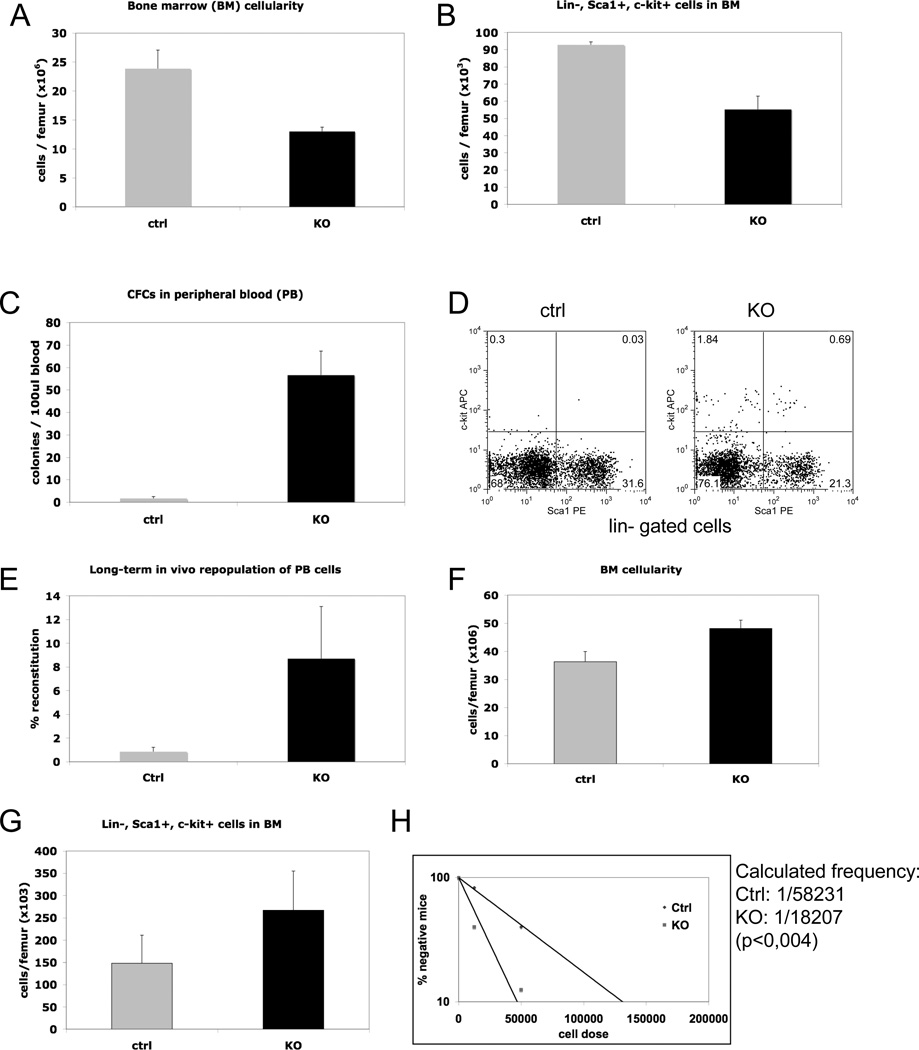
Four weeks following knockout induction, the Nf2 deficient mice exhibited a substantial (two-fold) reduction in BM cellularity compared to equally treated floxed littermate controls lacking the Cre transgene (Fig. 1A). This dramatic cell loss included the subset enriched for hematopoietic stem/progenitor cells (lin−, c-kit+, Sca1+) (Fig 1B). Concurrently we observed a large increase in the numbers of HS/PCs in the peripheral blood as measured by colony forming assays in vitro, phenotypic characterization by FACS as well as by competitive transplantation assays in vivo (Fig. 1C–E). Thus, induced Nf2 deficiency results in a rapid and substantial egress of HS/PCs from the BM into the bloodstream. The levels of circulating primitive cells declined somewhat after several months but were still clearly elevated 5 months after polyIC induction (Supplemental Fig. 3). This sustained mobilization effect did not appear to exhaust hematopoiesis in the knockout animals as they maintained intact levels of all blood lineages (data not shown) and showed a restored BM cellularity to normal levels after 5 months (Supplemental Fig 3). On the contrary, when the mice were analyzed 7 months after knockout induction, we observed an increased cellularity in the BM coupled with increased numbers of primitive LSK cells (Fig. 1, F and G). Moreover, transplantation experiments at limit dilution revealed a three-fold higher frequency of competitive repopulating units (CRU) in the knockout marrow (Fig. 1H). Thus despite a continuous outflow of primitive cells to the bloodstream, the Nf2 deficient mice develop a moderate increase in total cell content of the marrow and a substantial increase in the size of the stem cell pool. We concluded from these experiments that the Nf2 knockout mice may exhibit two distinct phenotypes affecting HSCs in bi-phasic manner: first one of mobilization and later one of enhanced generation.
Figure. 1. Rapid egress of HSCs and progenitor cells to the circulation and increase in HSC number over time following Nf2 deletion.
(A) Reduced BM cellularity in Nf2−/− mice 4 weeks after knockout induction (n=5, p<0,01). (B) Reduced numbers of primitive Lin−, Sca1+, c-kit+ cells in BM of Nf2 deficient mice (n=5, p<0,05). (C) Nf2−/− mice show enhanced numbers of progenitors in peripheral blood as determined by colony-forming-cell (CFC) assays (n=5, p<0,01). (D) Representative FACS plot showing the content of Lin−, Sca1+, c-kit+ cells in peripheral blood. (E) In vivo competitive repopulation assay to measure the content of stem cells in blood through competition with normal BM cells. Results show increased long-term (16 weeks) repopulating ability of peripheral blood cells from Nf2 deficient mice (n=4, p=0,02). (F) Increased BM cellularity in aged (7 months after polyIC induction) mutant mice (n=4, p<0.001). (G) Increased numbers of primitive hematopoietic cells determined by FACS (Lin−, Sca1+, c-kit+) in aged mutant mice (n=4, p<0,05). (H) Competitive repopulating unit (CRU) assays show an increased frequency of HSCs in the BM of aged mutant mice.
The HSC changes are cell non-autonomous and directly associated with changes in the bone marrow microenvironment
We next sought to determine the bases for the observed stem cell phenotypes, first whether they were intrinsic to the stem cells or due to alterations in the bone marrow microenvironment. To address this for the mobilization phenotype, we created two transplantation models by reconstituting either wild-type (WT) mice with Nf2 floxed BM cells or, conversely, Nf2 floxed mice with WT BM cells. When reconstituted after eight weeks, these mice were treated with polyIC to induce deletion of the floxed alleles. Interestingly, Nf2 knockout hematopoietic cells that had reconstituted a WT stroma environment did not show egress into the bloodstream. However, WT hematopoietic cells in an Nf2 knockout environment (four weeks after polyIC induction) displayed a similar mobilization phenotype as observed originally in the Nf2 knockout mice with a dramatic increase in CFC numbers in the blood (Fig. 2, A and B). Thus, the mobilization phenotype is specifically dependent on alterations in the bone marrow microenvironment. We further asked whether the increased mobilization could be coupled to defects in homing in Nf2 deficient animals. Indeed, when wild-type hematopoietic cells were intravenously infused to Nf2 deficient hosts, they showed markedly decreased homing to the marrow, while homing to the spleen was normal (Fig. 2, C and D). Taken together these experiments show that Nf2 deficiency in the BM microenvironment leads to both a retention defect, which increases mobilization, and reduced homing of HSC/Ps in a cell non-autonomous manner.
Figure 2. The HSC changes are cell non-autonomous and specifically dependent on alterations in the bone marrow microenvironement.
BM chimeras were generated by either transplanting Nf2 floxed BM cells into wildtype recipients or wildtype BM into Nf2 floxed recipients. Following hematopoietic reconstituion 8 weeks post-transplantion, the BM chimeras were treated with polyIC to induce gene deletion in Nf2 floxed cells. (A) Nf2 deficient progenitors that have reconstituted a wildtype environment do not show increased egress into the bloodstream as measured by CFC assays 4 weeks after polyIC induction (n=4 p=NS). (B) Wildtype cells in an Nf2 deficient environment display a similar dramatic retention defect as observed in the Nf2−/− mice (n=4, p<0,01). Fluorescently labeled wildtype BM cells were intravenously injected to lethally irradiated Nf2−/− or Nf2 flox/flox control mice. 16 hours later, mice were sacrificed and the numbers of homed cells in BM and Spleen were determined by FACS. (C) Reduced homing of c-kit + cells to the marrow space in Nf2 deficient mice (n=3, p=0,01). (D) No alterations in the homing to Spleen could be observed (n=3, p=NS). Lethally irradiated wildtype mice were reconstituted with Nf2 floxed BM cells and after 8 weeks treated with polyIC. 7 months later the BM was analyzed. (A) No alteration in BM cellularity in aged transplant recipients (n=4, p=NS). (B) Normal numbers of Nf2 deficient HSCs (Lin−, Sca1+, c-kit+, CD34− cells) in a wildtype environment. (n=4, p=NS).
Similarly, we determined the basis for the expansion of BM cells and increased stem cell pool in aged Nf2 mutant mice. To test whether this was due to an intrinsically mediated growth advantage in the Nf2 deficient HSC we transplanted wild-type mice with Nf2 floxed BM and, following full reconstitution, induced the gene deletion. Seven months after polyIC induction, we analyzed the BM and measured the stem cell content in the transplanted animals. Interestingly, we observed no significant differences in either the total cell content of the BM or in the numbers of phenotypically defined HSCs, (Fig. 2, E and F). Thus Nf2 deficiency does not affect stem cell homeostasis through cell autonomous mechanisms.
Expansion of stromal cells, elevated VEGF production and increased vascular structures in the bone marrow of Nf2 deficient mice
To further elucidate potential mechanisms behind the rapid egress of hematopoietic stem-and progenitor cells from the bone marrow, we analyzed bone sections from tibias taken 4 weeks after polyIC induction. Histological examination showed a prominent expansion of non-hematopoietic stromal cells distal to the cartilage zone of the epiphyseal plate in the Nf2 mutants (Fig. 3A). Consistent with this finding, we have also observed enhanced growth in vitro of Nf2 deficient stroma cells derived from primary bone marrow cultures (data not shown). The other prominent finding in the bone sections was a dramatic increase in the number of vessels in the marrow space of mutant tibias (Fig. 3B). At this stage, bone histomorphometry showed normal numbers of osteoblasts (Supplemental Fig. 4). Mobilization of HSC/Ps has been shown to be triggered by high levels of vascular endothelial growth factor (VEGF) (Hattori et al., 2001). This prompted us to examine the expression levels of VEGF in the Nf2 mutant mice since it could provide a mechanism for both the increased vessel formation and the mobilization phenotype. Indeed, expression of VEGF was markedly increased in the mutants, particularly in the areas of stromal cell expansion. (Fig. 3, C and D).
Figure 3. The Nf2 deficient mice display increased vascular structures and high VEGF production in the bone marrow, which may account for the retention and homing defect.
Mice were sacrificed 4 weeks after polyIC induction and the tibial bones were recovered and processed for histology. (A) Histology of tibias show a dramatic expansion of non-hematopoietic bone marrow stromal cells distal to the cartilage zone in the Nf2 mutants (between arrowheads). (B) Immunohistochemical staining with the endothelial marker CD31 reveals a large increase in the number of vessels in the marrow space of mutant tibias. In situ hybridization (C) and immunohistochemistry (D) show markedly increased expression levels of VEGF in tibia sections from the knockout mice particularly in areas of stromal cell expanion (between arrowheads).
We considered two potential mechanisms whereby VEGF could trigger mobilization. One would be a direct chemokinetic effect on the HSC/Ps and the other a secondary effect from the vascular remodeling. We assumed that a chemokinetic effect would be associated with changes either in the proliferation kinetics or in the expression of adhesion molecules. However, we did not detect any such changes in hematopoietic cells when assessing the cell cycle status in the primitive LSK cells by BrdU uptake (Supplemental Fig. 5) or when we measured expression levels of the adhesion molecules CXCR4 and integrin alpha 4 which are known key mediators of HSC retention in the BM (Supplemental Fig. 6). These findings suggest that the vascular changes per se are responsible for the impaired retention of HSC/Ps in the bone marrow.
Trabecular bone and osteoblast number increase in aged Nf2 deficient mice
We next sought to determine the mechanism behind the elevated HSC numbers in the aged mutant mice. Osteoblasts have been shown to have key regulatory functions for HSCs in the endosteal niche (Calvi et al., 2003; Visnjic et al., 2004; Zhang et al., 2003). Histological analysis of tibia sections from mice 7 months after polyIC induction showed a marked increase in trabecular bone in the mutants (Fig. 4A). Both the total bone area and the numbers of osteoblasts were elevated in the mutant animals (Fig. 4 B and C). It is possible that these changes have resulted in an expansion of osteoblastic HSC niches, which in turn could explain the increased numbers of stem cells in the aged mutant animals. Several other models have suggested that specific types of alterations that increase osteoblast number can increase HSCs (Calvi et al., 2003; Zhang et al., 2003).
Figure 4. Aged Nf2 deficient mice have increased trabecular bone and osteoblasts.
Mice were sacrificed 6–7 months after polyIC induction for BM analysis. (A) Tibial sections from the mutant mice showed a marked increase in trabecular bone structures (arrowheads). (B and C) Bone histomorphometry shows a marked increase in the relative bone volume (n=3, p<0,05) and in the number of osteoblasts (n=3, p<0,05) in the Nf2 mutant animals. (D) Cell cycle distribution of Lin−, Sca1+, c-kit+ cells measured by Hoechst and Pyronin Y staining. Representative FACS plots and graph showing accumulated data (n=3, p=NS). (E) Apoptosis assay using Annexin V staining on Lin−, Sca1+, c-kit+ cells. The lower right quadrant represents early apoptotic cells that are positive for Annexin V but negative for 7-AAD which labels dead cells. Representative FACS plots and graph showing accumulated data (n=3, p=NS).
To further explore the mechanism behind the increased stem cell numbers, we measured the viability and proliferation kinetics of HSCs in aged animals (6 months after polyIC treatment) using cell cycle and apoptosis assays. However, we found no difference in the cell cycle status or in the frequency of apoptotic LSK cells in Nf2 mutant mice (Fig. 4 D and E). This supports our previous conclusion that the phenotype is stem cell non-autonomous. It further suggests that the stem cell expansion is mediated by an overall increased niche capacity (i.e. a possibility to harbor more stem cells) rather than enhanced HSC stimulation from intrinsic or extrinsic factors.
DISCUSSION
We have found that Nf2/merlin plays a crucial role in regulating several components of the bone marrow microenvironment including stromal cells, endothelial cells and osteoblasts. Our model suggests that these effects on the microenvironment have severe consequences for the localization and number of bone marrow HSCs.
Merlin controls surface availability and signaling from various membrane receptors in different cell types and model organisms (Maitra et al., 2006; Curto et al., 2007). In vitro, Nf2-deficient primary osteoblasts are unable to silence EGFR signaling in response to intercellular contact, resulting in their continuous proliferation (Curto et al., 2007). Upon loss of Nf2, similar mechanisms might render endothelial cells, osteoblasts and stromal cells within the HSC niche unable to silence proliferative signals from locally acting growth factors such as VEGF causing the progressive expansion of the cellular components of niche. We therefore propose a model where loss of Nf2 leads to a rapid and substantial expansion of BM stroma cells expressing high levels of VEGF, which in turn triggers a strong angiogenic response and subsequently mobilization of HSC/Ps. Interestingly, it has been shown that systemic administration of VEGF triggers efficient HSC/P mobilization in a similar fashion to that observed in our mice (Hattori et al., 2001). The mobilization effect could theoretically be mediated by direct VEGF actions on the hematopoioetic cells but we found no alterations in cell cycle kinetics or in expression of adhesion molecules to support this. The role of VEGF in regulating vasculature is well established. VEGF induces endothelial cell proliferation and vascular remodeling but it also increases vessel permeability (Connolly et al., 1989; Senger et al., 1983). The latter effect is visualized by the presence of leaky vessels in transgenic mice overexpressing VEGF (Thurston et al., 1999). A likely mechanism for the mobilization phenotype observed in our mice is therefore a VEGF-mediated induction of more abundant and more permeable sinusoidal vessels in the bone marrow.
In addition to its’ role in vitro, merlin has also been implicated in the regulation of osteoblasts in vivo. Using Cre expressed in neural crest progenitors and Schwann cells it was shown that conditional Nf2 mutants develop Schwann cell hyperplasias as well as schwannomas, and osseous metaplasia (Giovannini et al., 2000). The latter effect was probably due to Nf2 deletion in neural crest derived osteoblasts (Giovannini et al., 2000). This in combination with the high incidence of osteosarcomas in heterozygous Nf2 mutants, where the second allele has been spontaneously deleted, indicates a critical role for merlin in regulation of osteoblast function (McClatchey et al., 1998). We did not observe any osteosarcomas during the life-span of our animals but the bone and osteoblast changes might be viewed as predisposing to tumor development. It is tempting to conclude that the bone phenotype in our model would be a direct consequence of Nf2 deletion in the osteoblasts. However, due to the late occurrence of the bone phenotype we cannot exclude a secondary effect where the increased vasculature plays a role as well. Indeed it has been shown that vascular remodeling induced by angiogenic factors such as VEGF is associated with increased osteogenesis at least during development (Gerber et al., 1999; Suzuki et al., 2007). It was recently demonstrated that these osteogenic effects of VEGF are osteoblast non-autonomous and secondary to the angiogenic response (Wang et al., 2007). To precisely determine the basis for the bone phenotype it will be necessary to generate mice with a cre specific for osteoblasts as well as endothelial cells.
Our data demonstrate profound and immediate effects on cell and tissue organization upon loss of merlin function in adult tissues. This highlights the importance of this gene, not only as a tumor suppressor, but as a key regulator of normal tissue homeostasis, including niche homeostasis. Nf2 deletion exemplifies the importance of the niche concept by highlighting the marked effects of microenvironment on stem cell localization and function. The relative cellular content, how the cells interact and how they are organized are all perturbed in the mutant mice we studied so we cannot conclude with precision which specific changes in the architectural organization of the marrow are critical. However, it is clear that modifying the non-hematopoietic components of bone marrow, profoundly alters hematopoietic stem cell localization and, ultimately, number.
MATERIALS AND METHODS
Mice
Nf2 floxed mice (a gift from Dr. M. Giovannini) were generated as described previously (Giovannini et al., 2000). The mice were backcrossed at least 4 generations on a C57/B6 background and crossed with C57B6 Mx1 Cre transgenic mice. Mice were bred in-house in a pathogen-free environoment. The Subcommittee on Research Animal Care of the Massachusetts General Hospital (MGH) approved all animal work according to federal and institutional policies and regulations. Mice and cell samples were genotyped using a 3 primer strategy to detect wildtype, floxed and deleted alleles of Nf2 simultaneously as described previously (Giovannini et al., 2000).
Cell culture
We performed colony assays by culturing cells in methylcellulose-containing medium M3434 (Stem Cell Technologies). To establish cultures of adherent stromal cells, BM cells were passaged in Myelocult medium (Stem Cell Technologies).
Flow cytometry and cell sorting
For lineage staining we used a cocktail of biotinylated anti-mouse antibodies to Mac-1α (CD11b), Gr-1(Ly-6G & 6C), Ter119 (Ly-76), CD3ε, CD4, CD8a (Ly-2), and B220 (CD45R) (BD Biosciences). For detection of HSC enriched poulations we used streptavidin conjugated with PE/Cy7, c-Kit-APC (CD117), Sca1-PE, CD34-FITC (all from BD Biosciences). For congenic strain discrimination anti-CD45.1-PE and anti-CD45.2 FITC antibodies (BD Biosciences) were used. For BrdU incorporation we used the FITC-BrdU Flow Kit (BD Biosciences) following administration of an admixture of 1 mg/ml of BrdU (Sigma) to drinking water for 3 days. For cell cycle analysis, cells were incubated with Hoechst (10µg/mL) in staining media (HBBS + 10% FCS) and subsequently stained for cell surface markers (same as above except that FITC conjugated Sca1 was used). Following fixation overnight (5% PFA) cells were stained with Pyronin Y and analyzed by flow cytometry. For the apoptosis assay we stained cells with lineage markers as above and with Pacific Blue conjugated Sca1 and FITC labeled c-kit. We then used 7-AAD and AnnexinV-APC according to the manufacturer’s instructions (BD Biosciences) to label and distinguish dead and early apoptotic cell fractions. For detection of adhesion molecules, FITC and PE conjugated antibodies against CXCR4 and integrin alpha4 respectively were used (BD Biosciences)
Transplantation Assays
For all transplantation assays the B6 congenic CD45.1 and CD45.2 strains were used to distinguish between test cells, competitor cells and host cells. Recipient mice were always lethally irradiated (9,5 Gy) and cells were transplanted intravenously through the tailvein. For transplantations to reconstitute animals prior to polyIC induction 2×106 whole bone marrow cells were injected. For the competitive repopulation assay with peripheral blood we used an equivalent of 200ul blood competed with 2×105 unfractionated bone marrow cells. For competitive repopulating unit (CRU) assays we used limiting numbers of test cells 2×105, 5×104, 1.25×104 competed against 2×105 support cells. Engraftment efficiency in recipients was monitored by the relative contribution of CD45.1, CD45.2 or CD45.1XCD45.2 cells using FACS analysis of peripheral blood. For the homing assasy, BM cells were labeled with 5 microM carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) (Molecular Probes Inc.) in accordance with the manufacturer's instructions. Labeled cells (2×107 per mouse) were injected and homing to BM and spleen measured by the presence CFDA-SE positive cells after 16 hours.
Bone histology and histomorphometry, in situ hybridization and immunohistochemistry
For histological analysis, tissues were fixed and stored as previously described (Chiusaroli et al., 2003). Hindlimbs were decalcified, and paraffin blocks were prepared by standard histological procedures. Static histomorphometry was performed on the same paraffin samples with an Osteomeasure system (Osteometrics Inc., Atlanta, GA), using standard procedures (Chiusaroli et al., 2003).
In situ hybridization was carried out as described previously (Chiusaroli et al., 2003) using complementary 35S-labeled riboprobes on paraffin sections of bone specimens prepared as above.
For immunohistochemistry, antigen retrieval was carried out with proteinase K (20mg/ml, Roche), followed by 3% H2O2 treatment to block endogenous peroxidase. The TSA Biotin system (PerkinElmer) was used according to the manufacturer’s instructions. Specimens were incubated with mouse anti-CD31 antibody (BD biosciences) or anti-VEGF antibody (Santa Cruz Biotechnology) for 1hr at room temperature.
Staistsical analysis
The results are expressed as the mean ± standard error of the mean (SEM) for n given samples. The Student t-test or Mann-Whitney’s test was used to determine the significance of results; significance was set at P values less than 0.05.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Marco Giovannini for providing the Nf2 floxed mice. This work was supported by a National Institutes of Health Grant (HL44581) to D.T.S and by an EMBO postdoctoral grant to J.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Chiusaroli R, Maier A, Knight MC, Byrne M, Calvi L, Baron R, Krane SM, Schipani E. Collagenase Cleavage of Type I Collagen is essential for both Basal and Parathyroid Hormone (PTH)/PTH-Related Peptide Receptor- Induced osteoclast activation and has differential effects on discrete bone compartments. Endocrinology. 2003;144:4106–4116. doi: 10.1210/en.2003-0254. [DOI] [PubMed] [Google Scholar]
- Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L, Woodruff JM, Berns A, Thomas G. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 2000;14:1617–1630. [PMC free article] [PubMed] [Google Scholar]
- Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003;17:1090–1100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Blank U, Helgadottir H, Bjornsson JM, Ehinger M, Goumans MJ, Fan X, Leveen P, Karlsson S. TGF-beta signaling-deficient hematopoietic stem cells have normal self-renewal and regenerative ability in vivo despite increased proliferative capacity in vitro. Blood. 2003;102:3129–3135. doi: 10.1182/blood-2003-04-1300. [DOI] [PubMed] [Google Scholar]
- Maitra S, Kulikauskas RM, Gavilan H, Fehon RG. The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol. 2006;16:702–709. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- McClatchey AI, Giovannini M. Membrane organization and tumorigenesis--the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–2277. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- McClatchey AI, Saotome I, Mercer K, Crowley D, Gusella JF, Bronson RT, Jacks T. Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev. 1998;12:1121–1133. doi: 10.1101/gad.12.8.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey AI, Saotome I, Ramesh V, Gusella JF, Jacks T. The Nf2 tumor suppressor gene product is essential for extraembryonic development immediately prior to gastrulation. Genes Dev. 1997;11:1253–1265. doi: 10.1101/gad.11.10.1253. [DOI] [PubMed] [Google Scholar]
- McLaughlin ME, Kruger GM, Slocum KL, Crowley D, Michaud NA, Huang J, Magendantz M, Jacks T. The Nf2 tumor suppressor regulates cell-cell adhesion during tissue fusion. Proc Natl Acad Sci U S A. 2007;104:3261–3266. doi: 10.1073/pnas.0700044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Miyamoto T, Fujita N, Ninomiya K, Iwasaki R, Toyama Y, Suda T. Osteoblast-specific Angiopoietin 1 overexpression increases bone mass. Biochemical and biophysical research communications. 2007;362:1019–1025. doi: 10.1016/j.bbrc.2007.08.099. [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.