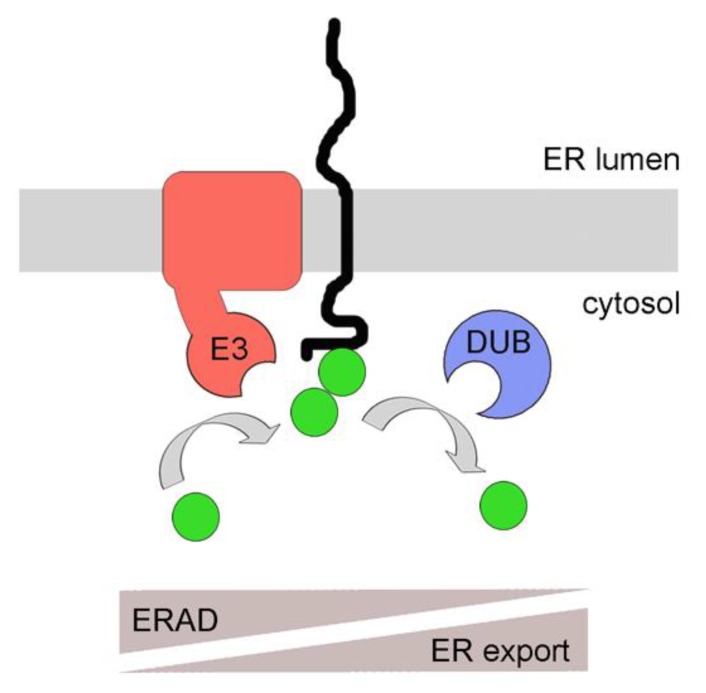
Figure 2.
A model for poly-ubiquitin chain (PUC) processing as a mechanism for coupling ER protein quality control with ERAD. Membrane proteins with exposed cytosolic domains are ubiquitinated by E3 ligases. Due to topological confinement of membrane proteins and membrane integrated E3 ligases to the same bilayer, ubiquitination might occur frequently, even on correctly folded proteins. DUBs can remove ubiquitins, favoring ER export of correctly folded species. Reoccurring ubiquitination due to prolonged ER retention as a result of misfolding (in the cytosol or ER lumen) will favor the assembly of PUCs to induce targeting to the proteasome, thereby favoring ERAD. A similar mechanism might occur for soluble proteins prior to their post-translational translocation across the ER membrane, a pathway called prERAD. See text for details. E3 = E3 ligase. DUB = deubiquitinase. Filled green circles: ubiquitin.