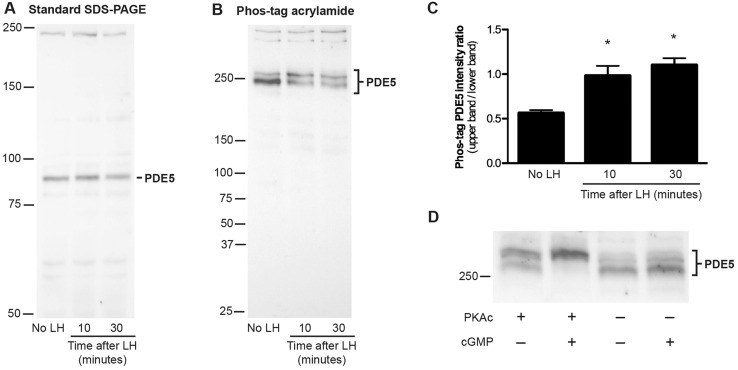
Fig. 5.
Rapid phosphorylation of PDE5 in follicles in response to LH signaling. (A,B) Western blots of proteins from follicles with or without 10 or 30 min of treatment with LH, which were probed with an antibody against PDE5. (A) Standard SDS-PAGE gel, 30 µg protein per lane; (B) SDS-PAGE gel containing 25 μM Phos-tag acrylamide, 20 µg protein per lane. Molecular weight standards are shown for reference, but do not indicate relative molecular mass on a Phos-tag gel. (C) Ratios of the intensity of the upper band to that of the lower band; mean±s.e.m. for four blots similar to that shown in B. (D) Phos-tag gel separation and immunoblotting for PDE5 in follicle lysates that had been incubated with or without the catalytic subunit of PKA (PKAc). Incubations were performed with or without 10 μM cGMP (see supplementary Materials and Methods); cGMP binds to an allosteric site on PDE5 and is required for PKAc to phosphorylate PDE5 (Corbin et al., 2000; Rybalkin et al., 2002). In the presence of PKAc and cGMP, the migration of PDE5 was retarded, indicating phosphorylation.