Abstract
Background
Insufficient data on neurodevelopmental benefits of antiretroviral therapy (ART) in children.
Methods
Prospective study of 329 mothers and children aged 0–6 years to assess neurodevelopment. Results stratified by the maternal (M) and child (C) HIV status (MHIV−/CHIV−, MHIV+/CHIV−, and MHIV+/CHIV+). Gross Motor, Visual Reception, Fine Motor, Receptive and Expressive Language scores assessed by Mullen Scales of Early Learning. Global cognitive function was derived from an Early Learning Composite score (ELC). Standardized Weight and Height for Age z-scores were constructed and the lowest 15% cutoff defined disability. Generalized linear models were used to estimate Prevalence Rate Ratios (PRR) adjusted for the child’s age, weight and height. In HIV-positive children, generalized linear models assessed the impact of ART initiation and duration on neurodevelopment.
Results
Compared to MHIV−/CHIV− children, HIV+ children were more likely to have global deficits in all measures of neurodevelopment except gross motor skills, whereas in MHIV+/CHIV− children, there was impairment in receptive language (adj.PRR=2.67, CI: 1·08, 6.60) and the ELC (adj.PRR=2.94, CI: 1.11, 7.82). Of the children born to HIV positive mothers, HIV+ children did worse than - MHIV+/CHIV− only in Visual Reception skills (adj.PRR=2.86; CI: 1.23–6.65). Of the 116 HIV+ children, 44% had initiated ART. Compared to ART duration of <12 months, ART durations 24–60 months was associated with decreased impairments in Fine Motor, Receptive Language, Expressive Language and ELC scores.
Conclusions
Longer duration on ART is associated with reduction of some neurologic impairment and early diagnosis and treatment of HIV+ children is a priority.
Keywords: HIV, neurodevelopment, HIV infected children, HIV affected children, ARTs
Introduction
Neurodevelopmental impairments are an important but largely unaddressed problem among children in low-income countries,1–3 but in the developed world, HIV has emerged as a leading cause of neurologic impairment4. HIV-infected children present with a wide range of disabilities, including generalized cognitive defects,5 motor deficits,6;7 and visual,8–10 language,7;11;12 or learning disorders7;13. These deficits are thought to result from the increased risk to central nervous system as the HIV virus can permeate the blood-brain barrier14.
A recent review on the impact of HIV infection on cognitive development in children found that of the fifty-four studies identified, over 75% of the studies were from the United States or Europe with results that are not generalizable to sub-Saharan Africa where a majority of HIV infected and exposed children live15. The review identified a significant gap in our knowledge about the impact of HIV infection on neurodevelopment outcomes among children from developing world settings but concluded that a majority of studies from developed nations found a detrimental neurocognitive impact as a result of HIV infection. Few studies in the developing world have assessed the impact of HIV infection on neurodevelopmental outcomes and results are mixed, with some studies showing no impact, 16 whereas the majority of studies 7,11,17;18 found that HIV-positive children had higher rates of cognitive and motor impairment compared with uninfected children. This in part may be due to differences in neurodevelopmental assessment, poor ART availability and adherence, differences in populations assessed as a majority of these studies were in urban settings, as well as the lack of prospective data where confounders could potentially be controlled for. Antiretroviral Therapy (ART) improves survival of HIV-infected children, and has been shown to reduce neurologic pathology,19;20 and HIV-infected children accessing care have improved motor development scores21. ART is becoming increasingly available in resource-poor settings, although little is known about the impact of age at initiation and duration of ART on neurodevelopment.
The majority of studies on the impact of HIV infection and ART on neurodevelopment outcomes among children in western countries are not applicable to developing countries due to differences in environmental exposure such as substance use, different child-rearing environments and other risk factors such as malnutrition and high levels of infectious diseases and opportunistic infections in developing country settings22. There are few prospective studies assessing long term neurocognitive outcomes in children in developing countries, most only follow children up to 24 months of age and are in hospital-based, urban settings which are not representative of the majority of children affected by HIV globally. We assessed neurodevelopmental outcomes among children infected and affected by HIV, and prospectively examined the impact of ART use on neurodevelopmental outcomes among HIV positive children in a rural African, community-based setting.
Methods
Mothers and children enrolled in this study were recruited from the Rakai Community Cohort Study (RCCS), the nevirapine (NVP) Prevention of Mother to Child HIV Transmission (PMTCT) study23, and from Rakai ART clinics providing free ART funded by the Presidents Emergency Plan for AIDS Relief (PEPFAR). Since 1994, RCCS has maintained approximately annual surveillance in an open, community-based cohort of ~14,000 adults aged 15 to 49 years in rural Rakai, Uganda24;25. Detailed information is collected on demographic, behavior, and health status. After the completion of the interview, blood is obtained for HIV serology. HIV testing used two separate EIAs (Vironestika, Organon Teknika and Welcozyme) with Western blot confirmation (HIV-1 Western Blot, Bio-Metriux-Vitek) of EIA discordant samples and of new seroconverters. HIV infection in children <18 months was detected by RNA-RT PCR using Roche Amplicor 1.5 assays (Branchberg, NJ), and by EIA/WB in older children.
Based on WHO guidelines26, all children less than 24 months had ART initiated upon diagnosis (AZT/3TC/Nevirapine or D4T/3TC/Nevirapine for children less than 3 years of age), for children between 2 and 5 years, ART was initiated when CD4% declined ≤ 25% and for children over the age of 5 years, ART was initiated at CD4 ≤ 350 cells/mm3 or at WHO clinical stage 3 or 4 disease.
Mothers gave written informed consent to be interviewed for: 1) the Ten Questions screening tool (TQ10) which is a mother/caretaker interview shown to be reliable, and valid (sensitivities 80–100%), for detection of serious cognitive, motor and seizure disorders 27 and 2) questionnaire to assess morbidity and mortality of the child. The questions for assessment of morbidity included asking the parent or guardian if the child had experienced any illness in the past 3 months and if yes, if the child had experienced the following in the past 3 months and the duration of the illness: fever, convulsions, fainting, cough, difficulty breathing, oral thrush, vomiting, non-bloody diarrhoea, bloody diarrhoea, jaundice, abnormal body movements, skin rash, boils, peeling of skin, passed worms in stool/vomiting worm, body swelling, silky hair, weight loss, loss of appetite, apathy and any other illness. For the child, maternal/guardian consent was obtained for: 1) anthropometric assessments (height in cms and weight in kilograms), and 2) neurodevelopmental assessments using the Mullen Scales of Early Learning for children under 6 years of age28. All HIV-negative children testing positive in the TQ10 screening test (108 out of 340) and all HIV-positive children were referred for in-depth neurodevelopmental assessments using the Mullen Scales of Early Learning.
The Mullen Scales of Early Learning test provides scores for Gross Motor, Visual Reception, Fine Motor, Receptive Language, and Expressive Language domains. It has excellent correspondence validity to the Bayley scales2 and has the advantage of estimating a global cognitive function from the Early Learning Composite (ELC) score. The ELC is derived as the average of the Z scores for visual reception, fine motor, receptive language and expressive language, which are converted into a standard score using a conversion table provided by the Mullens Learning test. A two week training of midwives in the use of the Mullen test was conducted by a psychologist and the tools were pilot tested among 2–3 children in each age category from 0 to 6 years of age.
Based on HIV test results, mother (M) and child (C) pairs formed three groups: reference group of HIV negative children born to HIV negative mothers (MHIV−/CHIV−); HIV affected children who are HIV negative and born to HIV positive mothers (MHIV+/CHIV−); HIV infected children born to HIV positive mothers (MHIV+/CHIV+). Comparison at baseline was made among MHIV+/CHIV+, MHIV+/CHIV−, and MHIV−/CHIV− to assess differences in neurodevelopmental outcomes. HIV positive children were followed prospectively to assess the impact of ART initiation and duration on neurodevelopment outcomes.
Statistical Methods
All Mullen cognitive scores were age standardized using a modified Mullen age-standardization based on the normal distribution of standardized neurodevelopmental scores for the HIV-negative children controlling for age. Predicted Z-scores were estimated by age for the HIV-positive and HIV-affected children. To determine a threshold for disability, z scores (based on control group of HIV-negative children) of either −1.5 or −2.029;30 have been used by studies. Using the standardized tables for the Mullen, the standard scores (from American norms in the Mullen table) for the referent MHIV−/CHIV− children equivalent to a z score of approximately the 15th percentile averaged across the various scales for the Ugandan MHIV−/CHIV− children was approximated at a z-score of <−1.5, and this cut-off was used to create a dichotomous disability score for each of the five Mullen domains.
Generalized linear models were used to estimate Prevalence Rate Ratios (PRR) to assess the potentially non-linear associations between HIV status and Mullen cognitive scores. All multivariate models adjusted for the child’s age, weight and height. 95% confidence intervals were generated using generalized estimating equation (GEE) models with robust variance with an exchangeable correlation matrix to account for correlation due to repeat observations in individual children.
In a sub-analysis of HIV-positive children, generalized linear models were fitted to assess the impact of ART on neurodevelopment scores, adjusting for age at ART initiation and ART duration. Early Learning Composite scores were plotted against ART duration (in months), adjusted for age, height and weight z-scores, current age, and study visit, and controlled for correlation due to multiple visits using generalized estimating equations (xtgee). All analyses were determined a priori and were performed using the statistical analysis software STATA version 12 (Stata Corp LP, College Station, TX).
This study was approved by Scientific and Ethics Committee of the Uganda Virus Research Institute, the Uganda National Council of Science and Technology in Uganda, and Johns Hopkins Bloomberg School of Public Health.
Results
329 mother-infant pairs were enrolled, of whom 108 (32·8%) were HIV-negative mothers and HIV-negative children (MHIV−/CHIV−), 105 (32·0%) in group B (HIV-positive mothers and HIV-negative children (MHIV+/CHIV−) and 116 (35·3%) in group C (HIV-positive mothers and HIV-positive children (MHIV+/CHIV+). Table 1 shows the sociodemographic characteristics at enrollment and the proportion of children classified with a neurodevelopmental impairment, stratified by the mother and child’s HIV status. On average, children in group A (MHIV−/CHIV− reference group) were significantly older compared to children in groups B or C. A significantly higher proportion of children in group C (HIV-positive children) were classified as impaired in all domains (except Gross Motor), and in the Early Learning Composite (ELC) score, compared to children in groups A or B.
Table 1.
Basic demographic information by maternal and child HIV Status
| ALL CHILDREN | Group A MHIV−/CHIV− N=108 |
Group B MHIV+/CHIV− N=105 |
Group C MHIV+/CHIV+ N=116 |
P-Value |
|---|---|---|---|---|
| Age, months | ||||
| Median | 57.5 | 36.1 | 49.6 | <0.001 |
| Mean | 49.5 | 39.7 | 47.9 | 0.003 |
| Gender | ||||
| Males | 47.2% | 51.4 % | 40.5% | 0.258 |
| Females | 52.8% | 48.6% | 59.5% | |
| WAZŦ(mean) | −1.44 | −1.56 | −1.73 | 0.150 |
| HAZŦ(mean) | −1.51 | −1.57 | −1.90 | 0.350 |
| Neurodevelopment Disability* | ||||
| Gross Motor | 25.0 % | 11.4 % | 23.3 % | 0.021 % |
| Fine motor | 10.2 % | 6.7 % | 19.8 % | 0.011 % |
| Visual Reception | 6.5 % | 5.7 % | 26.7 % | <0.001 % |
| Receptive Language | 6.5 % | 11.4 % | 21.6 % | 0.004 % |
| Expressive Language | 8.3 % | 9.5 % | 21.6 % | 0.007 % |
| ELC** | 5.6 % | 7.6 % | 24.1 % | <0.001 % |
A 15% cut-off of each age standardized z-score, based on mean neurodevelopment scores of HIV-negative children, was used to create a dichotomous disability score
ELC-Early Learning Composite Score
WAZ: Weight for Age Z-Score; HAZ: Height for Age Z-Score
The bivariate and multivariate PRR of a disability by maternal and child HIV status are summarized in Table 2. Adjusted analyses controlled for the child’s age at baseline, weight for age and height for age z-scores. In the unadjusted analyses, the PRR of a disability were significantly higher among HIV-positive children compared to the reference group A children in fine motor, visual reception, receptive and expressive language scores, and overall ELC score (PRR=4.34; CI: 1.87, 10.10). When HIV-infected children were compared to either MHIV−CHIV− or MHIV+CHIV− children, the PRR of disability was significantly higher for all neurologic domains and the overall ELC score. There were no consistent differences between Group B and A children.
Table 2.
Prevalence Risk Ratio of Disability by Child and Maternal HIV Status at Baseline
| Disability Assessment (A) HIV−M/HIV−C (N=108) (B)HIV+M/HIV−C (N=105) (C)HIV+M/HIV+C (N=116) |
Bivariate PRR (CI) | Multivariate ‡ PRR (CI) |
|---|---|---|
| Gross Motor Score | ||
| B vs A | 0.46 (0.24,0.86) | 0.59(0.31,1.11) |
| C vs A | 0.93(0.58,1.48) | 0.87(0.55,1.39) |
| C vs B | 2.04(1.09,3.82) *** | 1.24(0.68,2.27) |
| Fine Motor Score | ||
| B vs A | 0.65(0.26,1.63) | 1.00(0.36,2.73) |
| C vs A | 1.95(1.00,3.81) ** | 2.39(1.15,4.95) ** |
| C vs B | 2.97(1.33,6.66) *** | 1.99(0.90,4.41) |
| Visual Reception Score | ||
| B vs A | 0.88(0.31,2.54) | 1.61(0.59,4.39) |
| C vs A | 4.12(1.89,8.98) ** | 5.86(2.30,14.92) ** |
| C vs B | 4.68(2.03,10.78) *** | 2.86(1.23,6.65) *** |
| Receptive Language Score | ||
| B vs A | 1.76(0.72,4.31) | 2.67(1.08,6.60) * |
| C vs A | 3.33(1.50,7.38) ** | 4.20(1.83,9.64) ** |
| C vs B | 1.89(1.00,3.57) *** | 1.45(0.80,2.64) |
| Expressive Language Score | ||
| B vs A | 1.14 (0.48,2.71) | 1.19(0.52,2.71) |
| C vs A | 2.59(1.26,5.30) ** | 2.27(1.15,4.50) ** |
| C vs B | 2.26(1.14,4.49) *** | 1.70(0.77,3.76) |
| ELC score | ||
| B vs A | 1.37(0.49,3.83) | 2.94(1.11,7.82) * |
| C vs A | 4.34(1.87,10.10) ** | 6.87(2.54,18.58) ** |
| C vs B | 3.17(1.51,6.65) *** | 1.82(0.90,3.67) |
Adjusting for HAZ (Height for Age Z-Score), WAZ (Weight for Age Z-Score), and Age at baseline
HIV affected children (group B) significantly different from reference children (group A)
HIV infected children (group C) significantly different from reference children (group A)
HIV infected children (group C) significantly different from affected children (group B)
In the adjusted analyses, compared to children in group A, HIV-infected children had significant impairment in Fine Motor (adj.PRR=2·39; CI: 1.15–4.95), Visual Reception (adj.PRR=5·86, CI:2.30–14.92), Receptive Language (adj.PRR= 4.20; CI: 1.83–9.64), Expressive Language (adj.PRR=2·27; CI: 2.27–1.15–4.50) and ELC scores (adj. PRR=6.87; CI: 2.54–18.58). HIV-infected children (Group C) had poorer scores than children affected with HIV (group B) in Visual Reception scores (adj. PRR=2.86). When comparing MHIV+/CHIV− to MHIV−/CHIV− children, there were no significant differences in neurodevelopmental scores except for Receptive Language (adj.PRR=2.67) and ELC scores (adj. PRR=2.94, CI: 1.11, 7.82).
Of the 116 HIV-positive children, 51 (44%) received ART. Compared to HIV-positive children not on ART, those who initiated ART were significantly younger (median age 33·6 months versus 58·3 months, respectively, p=0·001). Of the children on ART, 19 (37·3%) had initiated ART between 0 and 23 months (only 5 initiated less than 12 months), 25 (49·0%) between 24 and 59 months and 7(13·7%) between the ages of 60 and 83 months. HIV-positive children on ART were assessed longitudinally for neurodevelopment outcomes at baseline, 12 months, 18 months, and 24 months. Of the 51 children, 83% were seen at the 12 month visit, 69% at the 18 month visit and 39% at the 24 month visit.
The PRRs of neurodevelopment disability by ever use of ART for 116 HIV-positive children with a total of 308 visits are summarized in Table 3. Of the 116 HIV positive children, the 65 not on ART contributed 160 visits while the 51 who initiated ART contributed 148 visits. Children who initiated ART were significantly more likely to have poorer scores for Visual Reception (adj. PRR=2.07, CI: 1.3, 3.3), Receptive Language (adj.PRR=2.47, CI: 1.6, 3.8), Expressive Language (adj.PRR=3.0, CI: 1.9, 4.9), and ELC scores (adj. PRR=1.93, CI: 1.3, 3.0).
Table 3.
Neurodevelopment Disability Among HIV-Positive Children by Ever Initiation of ART
| N=116 | Gross motor PRR* (95% CI) | Fine motor PRR* (95% CI) | Visual Reception PRR* (95% CI) | Receptive language PRR* (95% CI) | Expressive language PRR* (95% CI) | Early Learning Composite score |
|---|---|---|---|---|---|---|
| ART USE | ||||||
| Never Initiated (N=65) | 1 | 1 | 1 | 1 | 1 | 1 |
| Ever Initiated (N=51) | 1.36(0.9,2.0) | 1.45(0.9,2.3) | 2.07(1.3,3.3) | 2.47(1.6,3.8) | 3.0 (1.9,4.9) | 1.93 (1.3,3.0) |
| Current Age at study visit | 1.44(1.2,1.8) | 1.58(1.3,1.9) | 1.55(1.3,1.9) | 2.11(1.6,2.7) | 1.32(1.1,1.6) | 1.97(1.6,2.4) |
| HAZ | 0.69(0.6,0.9) | 0.87(0.7,1.1) | 0.71(0.6,0.9) | 1.03(0.9,1.1) | 0.85(0.7,1.1) | 0.76(0.6,0.9) |
| WAZ | 0.87(0.7,1.2) | 0.89(0.7,1.1) | 0.95(0.7,1.2) | 0.73(0.6,1.0) | 0.76(0.6,1.0) | 0.81(0.6,1.0) |
| Study visit | 1.0(1.0,1.0) | 1.0(1.0,1.0) | 0.97(0.9,1.0) | 0.97(1.0,1.0) | 0.99(1.0,1.0) | 0.98(1.0,1.0) |
adjusted for age, HAZ (Height for Age Z-Score), WAZ (Weight for Age Z-Score) and study visit
Generalized linear models were fitted (controlling for child’s age, height and weight z scores) to assess the impact of ART duration on risk of neurologic impairment among 51 HIV- positive children with 131 visits (Table 4). Children who were on ARTs for more than 24 months were significantly less likely to have neurologic impairment than children on ART for less than 24 months for Fine Motor (adj. PRR=0.15; CI: 0.01–0.5), Receptive Language (adj.PRR=0.38; 0.2–0.8), Expressive Language (adj.PRR=0.09; 0.01–0.3) and ELC scores (adj.PRR=0.45; CI: 0.1–0.15). Once the age of child and ART duration were controlled for, there was no statistically significant impact of age at ART initiation on neurologic outcomes.
Table 4.
Impact of ART Duration on Neurologic Impairment among HIV Positive Children
| N=51 | Gross Motor PRR (95% CI) | Fine Motor PRR (95% CI) | Visual Reception PRR (95% CI) | Receptive Language PRR (95% CI) | Expressive Language PRR (95% CI) | Early Learning Composite Score PRR (95% CI) |
|---|---|---|---|---|---|---|
| ART Duration (months) | ||||||
| 0–12 | 1 | 1 | 1 | 1 | 1 | 1 |
| 13–24 | 0.62(0.3,1.3) | 0.87(0.4,1.8) | 0.92(0.4,2.2) | 0.87(0.5,1.5) | 0.65(0.3,1.2) | 1.21(0.7,2.2) |
| 25–60 | 0.38(0.1,1.6) | 0.15(0.01,0.5)** | 0.52(0.1,2.3) | 0.38(0.2,0.8)* | 0.09(0.01,0.3)** | 0.45(0.1,0.15)* |
| Current Age (years) | 1.57(0.9,2.8) | 2.7(1.7,4.4)** | 1.59(0.9,2.9) | 2.44(1.5,4.1)** | 1.99(1.2,3.2)** | 2.2(1.2,3.9)** |
| Age at ART Initiation (years) | 0.89(0.5,1.5) | 0.63(0.4,1.0) | 1.05(0.6,1.9) | 0.87(0.6,1.3) | 0.68(0.4,1.1) | 0.96(0.6,1.5) |
PRR adjusted for age, HAZ (Height for Age Z-Score), WAZ (Weight for Age Z-Score) and study visit
p<0.05,
p<0.001
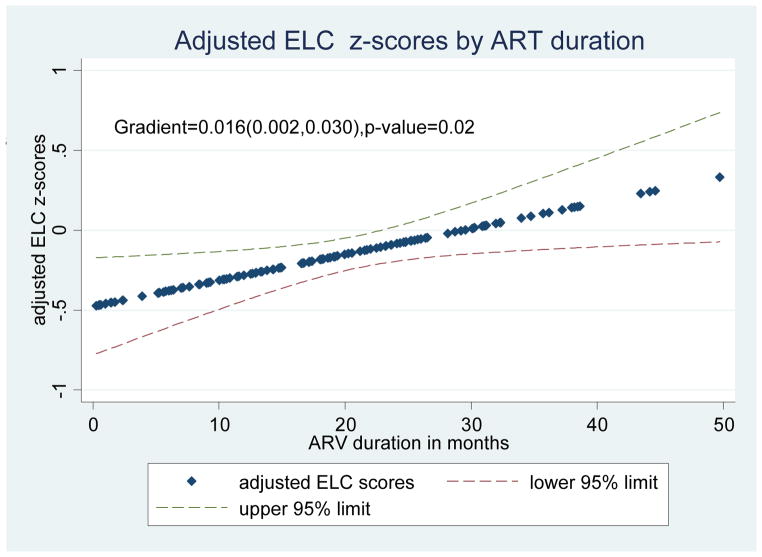
Figure 1 summarizes the Early Learning Composite scores plotted against ART duration (in months) and shows that on average, ELC scores improved by 0.016 per month duration of ART (gradient=0.016, p=0.02).
Figure 1. Adjusted Early Learning Composite Z-scores for HIV Positive Children, by ART duration.
* adjusted for HAZ (Height for Age Z-Score), WAZ (Weight for Age Z-Score), current age and study visit
Discussion
We assessed neurocognitive function in 3 groups of children less than 6 years of age, born to HIV negative and positive mothers using the Mullens Scales of Early Learning28. The key findings were that compared to HIV-negative children, HIV-infected children had deficits in all measures of neurodevelopment, whereas among HIV-negative children born to HIV+ mothers, only receptive language scores were significantly worse. The risk of overall cognitive impairment was significantly higher among children born to HIV-positive mothers (irrespective of the child’s HIV infection status), compared to the HIV-negative referent children. Among HIV-positive children, longer duration on ARTs was associated with a reduced risk of impairment in Fine Motor, Receptive Language, Expressive Language and overall global Early Learning Composite score.
A review of six studies assessing pediatric HIV and neurodevelopment in sub-Saharan Africa17 showed that motor development impairment was most commonly associated with HIV infection in young children, but concluded that there is an urgent need for data for children over 24 months of age. Our study confirms findings of significant neurodevelopmental impairment associated with HIV infection in children13;31.
Global cognitive defects were probably due to the direct effects of the virus on the brain areas associated with cognitive development and motor skills32. The HIV virus is present in the central nervous system (CNS) of most infected children, irrespective of age, CD4 count, or stage of disease33. The resulting inflammation leading to neuronal death is hypothesized to cause neurodevelopmental deficits.
Language development, and specifically receptive language, appears to be significantly impacted among both HIV-infected and affected children in our study as is seen in other studies34;35. In an American cohort of HIV exposed but uninfected children35, with a majority having in utero exposure to combination ARVs, high rates of late language emergence were identified in one (26%) and two (23%) year olds compared to unexposed controls. However once confounders were adjusted for, late language emergence was not associated with maternal combination ARV use but rather related to HIV exposure in utero. This finding is comparable to a an earlier study by Rice, et al34 which found high rates of language impairment among children infected and affected by HIV. In our study, children affected by HIV had no significant deficits compared to HIV-negative controls except for receptive language scores. Receptive language is more dependent on the working memory of the child in response to the items being presented, and may be a consequence of maternal illness and lack of an environment conducive to language learning. Although there is improvement in language skills after ART use in some studies36, others find no impact of ART on reversal of language delay, despite benefits in overall cognitive performance with extended ART use37. We found significant improvements in both Receptive and Expressive Language scores with extended ART use among HIV+ children. Impaired verbal performance can have a significant impact on academic performance as well as adherence to HIV treatment, and hence, interventions to enhance language acquisition are critical.
Based on the CHER randomized trial in South Africa which found improved survival among HIV-infected infants who initiated ART early,38 WHO changed its recommendation to initiate ART among all HIV positive children less than 2 years of age, irrespective of CD4 count or WHO clinical stage26. Approximately 56% of the infected children in our study initiated ART after 24 months of age. A majority of HIV positive children in Rakai are frequently brought to the ART clinic when the child is already sick and early ART initiation, especially for infants is challenging. As seen in table 3, ART initiation was associated with poorer neurologic outcomes most likely because a majority of children are already ill and hence ART initiation may be a proxy for advanced HIV disease. Unlike other studies that found no improvement in cognitive deficits in response to ART39;40, we found significant improvements in several neurodevelopmental domains with longer duration of ART use.
Interventions with proven efficacy to prevent or reverse neurodevelopmental impairment due to HIV are urgently needed. However studies assessing the impact of ART initiation on neurodevelopment outcomes are mixed: results from the PREDICT neurodevelopment study of HIV infected Thai and Cambodian children over a year old, randomized to early versus deferred ART treatment found that regardless of whether children initiated ART at CD4 15%–24% or deferred until CD4 was <15%, there was not significant impact on neurodevelopment outcomes assessed, despite the early ART arm achieving higher CD4 levels and viral suppression 41. The study concluded that cognitive deficits in HIV positive children potentially occur in infancy and they do not improve with initiation of ART after one year of age. However, another study of HIV-positive children aged 18 to 71 months21 found that early initiation of primary HIV care and ART, resulted in improved motor development and possibly cognitive development. Our study supports this observation. Other interventions that have shown some success have been reported recently; a study among Ugandan children 2 to 4 years of age using the Mullen instrument found significant improvements for a year-long caregiver training program to enrich the children’s home environment, specifically in receptive and expressive language. So although the HIV exposed but uninfected and HIV-positive children were at risk for significant language delay and impairment, the Mullen documented significant improvements associated with improved caregiving in early childhood42.
Some of the limitations of our study are that a majority of the infants and children were not enrolled at birth and did not initiate ART early. In addition, data on adverse birth outcomes were not available. It was also not possible to control for other risk factors for neurodevelopmental impairment such as malaria, as well as other environmental and parent or guardian factors. Also, we were unable to follow up all children who started on ART.
Conclusions
HIV infection in children resulted in global deficits in neurodevelopmental and cognitive function and extended ART use potentially mitigated some of the neurodevelopmental deficits. Interventions to prevent or reverse the negative consequences of HIV infection on neurodevelopmental outcomes of infants and children are critically needed in resource-poor settings.
Acknowledgments
Funding Sources:
Funding for this study was provided by Fogarty International-NICHD (5K01TW007403), Center for Global Health, Johns Hopkins University, and the WW Smith Charitable Trust Foundation.
The authors acknowledge the significant contribution of the field workers in implementing this study and thank the mothers and children who participated in this study
Footnotes
- International Conference on AIDS and STDs in Africa, Cape Town. 2013. Prospective study on neurodevelopmental benefits of ART in Children 0–6 years of age. Brahmbhatt, H.*, Bovin, M., Sempijja, V., Matovu, I., Kigozi, G., Serwadda, D., Gray, R.
- International AIDS Society (IAS), Rome. 2011. Impact of HIV and ARVs on Neurodevelopment in Children. Brahmbhatt, H*., Boivin, M., Ssempijja, V., Kagaayi, J., Matovu, J., Ssebagala, D., Sekasanvu, J., Nakafeero, M., Gray, R.
- XVII Conference on Retroviruses and Opportunistic Infections, San Francisco. 2010. Brahmbhatt, H.*, Kagaayi, J., Boivin, M., Matovu, I., Kigozi, G., Wabwire-Mangen, F., Gray, R. Association Between HIV Infection, ARV use and Neurodevelopmental Outcomes among Children Aged 0 to 14 Years in Rural Uganda.
Reference List
- 1.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369(9556):145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 2.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369(9555):60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engle PL, Black MM, Behrman JR, Cabral de MM, Gertler PJ, Kapiriri L, et al. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet. 2007;369(9557):229–242. doi: 10.1016/S0140-6736(07)60112-3. [DOI] [PubMed] [Google Scholar]
- 4.IOM. Neurological, psychiatric, and developmental disorders: Meeting the challenge in the developing world. Washington DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 5.Blanchette N, Smith ML, Fernandes-Penney A, King S, Read S. Cognitive and motor development in children with vertically transmitted HIV infection. Brain Cogn. 2001;46(1–2):50–53. doi: 10.1016/s0278-2626(01)80032-4. [DOI] [PubMed] [Google Scholar]
- 6.Epstein LG, Sharer LR, Oleske JM, Connor EM, Goudsmit J, Bagdon L, et al. Neurologic manifestations of human immunodeficiency virus infection in children. Pediatrics. 1986;78(4):678–687. [PubMed] [Google Scholar]
- 7.Boivin MJ, Green SD, Davies AG, Giordani B, Mokili JK, Cutting WA. A preliminary evaluation of the cognitive and motor effects of pediatric HIV infection in Zairian children. Health Psychol. 1995;14(1):13–21. doi: 10.1037//0278-6133.14.1.13. [DOI] [PubMed] [Google Scholar]
- 8.Drotar D, Olness K, Wiznitzer M, Guay L, Marum L, Svilar G, et al. Neurodevelopmental outcomes of Ugandan infants with human immunodeficiency virus type 1 infection. Pediatrics. 1997;100(1):E5. doi: 10.1542/peds.100.1.e5. [DOI] [PubMed] [Google Scholar]
- 9.Blanchette N, Smith ML, King S, Fernandes-Penney A, Read S. Cognitive development in school-age children with vertically transmitted HIV infection. Dev Neuropsychol. 2002;21(3):223–241. doi: 10.1207/S15326942DN2103_1. [DOI] [PubMed] [Google Scholar]
- 10.Tardieu M, Mayaux MJ, Seibel N, Funck-Brentano I, Straub E, Teglas JP, et al. Cognitive assessment of school-age children infected with maternally transmitted human immunodeficiency virus type 1. J Pediatr. 1995;126(3):375–379. doi: 10.1016/s0022-3476(95)70451-5. [DOI] [PubMed] [Google Scholar]
- 11.Msellati P, Lepage P, Hitimana DG, Van Goethem C, Van de PP, Dabis F. Neurodevelopmental testing of children born to human immunodeficiency virus type 1 seropositive and seronegative mothers: a prospective cohort study in Kigali, Rwanda. Pediatrics. 1993;92(6):843–848. [PubMed] [Google Scholar]
- 12.Nozyce M, Hittelman J, Muenz L, Durako SJ, Fischer ML, Willoughby A. Effect of perinatally acquired human immunodeficiency virus infection on neurodevelopment in children during the first two years of life. Pediatrics. 1994;94(6 Pt 1):883–891. [PubMed] [Google Scholar]
- 13.Levenson RL, Jr, Mellins CA, Zawadzki R, Kairam R, Stein Z. Cognitive assessment of human immunodeficiency virus-exposed children. Am J Dis Child. 1992;146(12):1479–1483. doi: 10.1001/archpedi.1992.02160240089028. [DOI] [PubMed] [Google Scholar]
- 14.Belman A. HIV-1 infection and AIDS. Neurologic Clinics. 2002;20(4):983–1011. doi: 10.1016/s0733-8619(02)00019-1. [DOI] [PubMed] [Google Scholar]
- 15.Sherr L, Mueller J, Varrall R. A systematic review of cognitive development and child human immunodeficiency virus infection. Psychol Health Med. 2009;14(4):387–404. doi: 10.1080/13548500903012897. [DOI] [PubMed] [Google Scholar]
- 16.Bagenda D, Nassali A, Kalyesubula I, Sherman B, Drotar D, Boivin MJ, et al. Health, neurologic, and cognitive status of HIV-infected, long-surviving, and antiretroviral-naive Ugandan children. Pediatrics. 2006;117(3):729–740. doi: 10.1542/peds.2004-2699. [DOI] [PubMed] [Google Scholar]
- 17.Abubakar A, Van BA, Van dV, Holding P, Newton CR. Paediatric HIV and neurodevelopment in sub-Saharan Africa: a systematic review. Trop Med Int Health. 2008;13(7):880–887. doi: 10.1111/j.1365-3156.2008.02079.x. [DOI] [PubMed] [Google Scholar]
- 18.McGrath N, Fawzi WW, Bellinger D, Robins J, Msamanga GI, Manji K, et al. The timing of mother-to-child transmission of human immunodeficiency virus infection and the neurodevelopment of children in Tanzania. Pediatr Infect Dis J. 2006;25(1):47–52. doi: 10.1097/01.inf.0000195638.80578.e0. [DOI] [PubMed] [Google Scholar]
- 19.Raskino C, Pearson DA, Baker CJ, Lifschitz MH, O’Donnell K, Mintz M, et al. Neurologic, neurocognitive, and brain growth outcomes in human immunodeficiency virus-infected children receiving different nucleoside antiretroviral regimens. Pediatric AIDS Clinical Trials Group 152 Study Team. Pediatrics. 1999;104(3):e32. doi: 10.1542/peds.104.3.e32. [DOI] [PubMed] [Google Scholar]
- 20.Laughton B, Cornell M, Grove D, Kidd M, Springer PE, Dobbels E, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS. 2012;26(13):1685–1690. doi: 10.1097/QAD.0b013e328355d0ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van RA, Dow A, Mupuala A, Stewart P. Neurodevelopmental trajectory of HIV-infected children accessing care in Kinshasa, Democratic Republic of Congo. J Acquir Immune Defic Syndr. 2009;52(5):636–642. doi: 10.1097/QAI.0b013e3181b32646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van RA, Harrington PR, Dow A, Robertson K. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: a global perspective. Eur J Paediatr Neurol. 2007;11(1):1–9. doi: 10.1016/j.ejpn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Kagaayi J, Dreyfuss ML, Kigozi G, Chen MZ, Wabwire-Mangen F, Serwadda D, et al. Maternal self-medication and provision of nevirapine to newborns by women in Rakai, Uganda. J Acquir Immune Defic Syndr. 2005;39(1):121–124. doi: 10.1097/01.qai.0000148530.66587.7c. [DOI] [PubMed] [Google Scholar]
- 24.Wawer MJ, Sewankambo NK, Serwadda D, Quinn TC, Paxton LA, Kiwanuka N, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group [see comments] Lancet. 1999;353(9152):525–535. doi: 10.1016/s0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 25.Gray RH, Wabwire-Mangen F, Kigozi G, Sewankambo NK, Serwadda D, Moulton LH, et al. Randomized trial of presumptive sexually transmitted disease therapy during pregnancy in Rakai, Uganda. Am J Obstet Gynecol. 2001;185(5):1209–1217. doi: 10.1067/mob.2001.118158. [DOI] [PubMed] [Google Scholar]
- 26.WHO. Antiretroviral therapy for HIV infection in infants and children:Towards universal access. 2010 [PubMed] [Google Scholar]
- 27.Durkin MS, Wang W, Shrout PE, Zaman SS, Hasan ZM, Desai P, et al. Evaluating a ten questions screen for childhood disability: reliability and internal structure in different cultures. J Clin Epidemiol. 1995;48(5):657–666. doi: 10.1016/0895-4356(94)00163-k. [DOI] [PubMed] [Google Scholar]
- 28.Mullen EM. Mullen scales of early learning: AGS Edition. Circle Pines, MN: American Guidance Services, Inc; 1995. [Google Scholar]
- 29.John CC, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122(1):e92–e99. doi: 10.1542/peds.2007-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boivin MJ, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, et al. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics. 2007;119(2):e360–e366. doi: 10.1542/peds.2006-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ultmann MH, Diamond GW, Ruff HA, Belman AL, Novick BE, Rubinstein A, et al. Developmental abnormalities in children with acquired immunodeficiency syndrome (AIDS): a follow-up study. Int J Neurosci. 1987;32(3–4):661–667. doi: 10.3109/00207458709043320. [DOI] [PubMed] [Google Scholar]
- 32.Ensoli F, Fiorelli V. HIV-1 Infection and the Developing CNS. NeuroAids. 2000;3(1) [Google Scholar]
- 33.George R, Andronikou S, du PJ, du Plessis AM, Van TR, Maydell A. Central nervous system manifestations of HIV infection in children. Pediatr Radiol. 2009;39(6):575–585. doi: 10.1007/s00247-009-1170-4. [DOI] [PubMed] [Google Scholar]
- 34.Rice ML, Buchanan AL, Siberry GK, Malee KM, Zeldow B, Frederick T, et al. Language impairment in children perinatally infected with HIV compared to children who were HIV-exposed and uninfected. J Dev Behav Pediatr. 2012;33(2):112–123. doi: 10.1097/DBP.0b013e318241ed23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rice ML, Zeldow B, Siberry GK, Purswani M, Malee K, Hoffman HJ, et al. Evaluation of risk for late language emergence after in utero antiretroviral drug exposure in HIV-exposed uninfected infants. Pediatr Infect Dis J. 2013;32(10):e406–e413. doi: 10.1097/INF.0b013e31829b80ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coplan J, Contello KA, Cunningham CK, Weiner LB, Dye TD, Roberge L, et al. Early language development in children exposed to or infected with human immunodeficiency virus. Pediatrics. 1998;102(1):e8. doi: 10.1542/peds.102.1.e8. [DOI] [PubMed] [Google Scholar]
- 37.Wolters PL, Brouwers P, Civitello L, Moss HA. Receptive and expressive language function of children with symptomatic HIV infection and relationship with disease parameters: a longitudinal 24-month follow-up study. AIDS. 1997;11(9):1135–1144. doi: 10.1097/00002030-199709000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith L, Adnams C, Eley B. Neurological and Neurocognitive Function of HIV-infected Children Commenced on Antiretroviral Therapy. SA Journal of Child Health. 2008;2(3):108–113. [Google Scholar]
- 40.Lindsey JC, Malee KM, Brouwers P, Hughes MD. Neurodevelopmental functioning in HIV-infected infants and young children before and after the introduction of protease inhibitor-based highly active antiretroviral therapy. Pediatrics. 2007;119(3):e681–e693. doi: 10.1542/peds.2006-1145. [DOI] [PubMed] [Google Scholar]
- 41.Puthanakit T, Ananworanich J, Vonthanak S, Kosalaraksa P, Hansudewechakul R, van der Lugt J, et al. Cognitive Function and Neurodevelopmental Outcomes in HIV-infected Children Older Than 1 Year of Age Randomized to Early Versus Deferred Antiretroviral Therapy: The PREDICT Neurodevelopmental Study. Pediatr Infect Dis J. 2013;32:501–508. doi: 10.1097/INF.0b013e31827fb19d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boivin MJ, Bangirana P, Nakasujja N, Page CF, Shohet C, Givon D, et al. A year-long caregiver training program to improve neurocognition in preschool Ugandan HIV-exposed children. J Dev Behav Pediatr. 2013;34(4):269–278. doi: 10.1097/DBP.0b013e318285fba9. [DOI] [PMC free article] [PubMed] [Google Scholar]