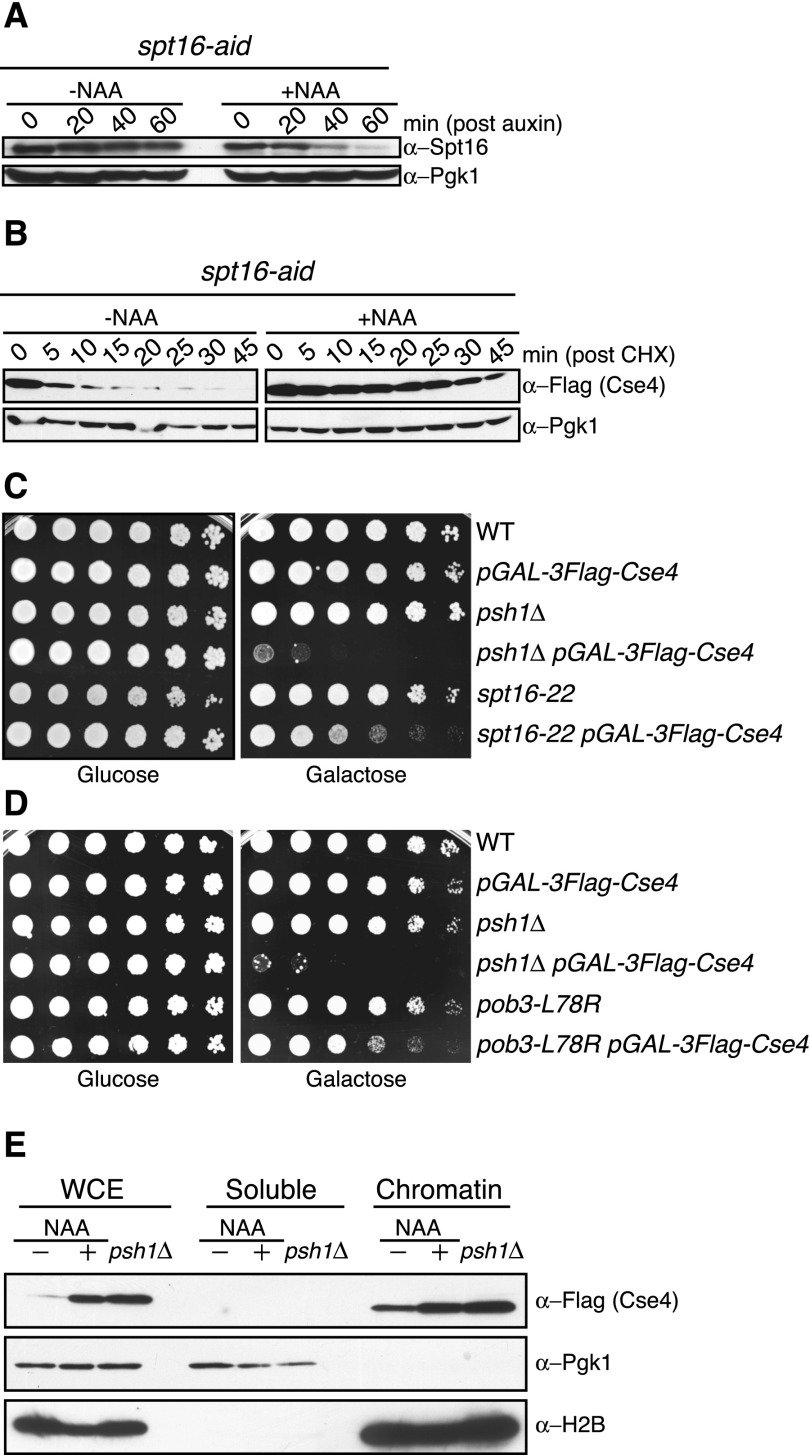
Figure 1.
The FACT complex facilitates CENP-ACse4 degradation in vivo. (A) spt16-aid (SBY11265) cells were treated with vehicle (95% ethanol [−NAA]) or auxin (+NAA) at time 0, and lysates were analyzed for Spt16-AID levels using α-Spt16 antibodies at the indicated time points. Pgk1 is shown as a loading control. (B) spt16-aid (SBY11268) cells containing pGAL-3Flag-CSE4 were treated with vehicle (−NAA) or auxin (+NAA) to degrade Spt16 for 1 h, followed by a 1-h galactose induction of 3Flag-Cse4. Protein synthesis was inhibited by cycloheximide (CHX) addition at time 0, and lysates were monitored for 3Flag-Cse4 levels with α-Flag antibodies at the indicated time points. Pgk1 is a loading control. Quantification of the stability is in Supplemental Figure S1. (C) Fivefold serial dilutions of the indicated strains (SBY3, SBY8851, SBY8336, SBY8903, SBY6006, and SBY11186) were plated on glucose or galactose medium at 30°C. (D) Fivefold dilutions of the indicated strains (SBY3, SBY8851, SBY8336, SBY8903, SBY11295, and SBY11296) were plated on glucose or galactose medium at 23°C. (E) pGAL-3Flag-CSE4 was overexpressed with galactose in either spt16-aid (SBY11268) cells treated with (+NAA) or without (−NAA) auxin as in B or psh1∆ (SBY8903) cells. This was followed by cycloheximide addition for 30 min, and then whole-cell extracts (WCE) were fractionated into soluble and chromatin fractions. Cse4 levels were monitored in each fraction with α-Flag antibodies. Pgk1 and H2B are markers of the soluble and chromatin fractions, respectively.