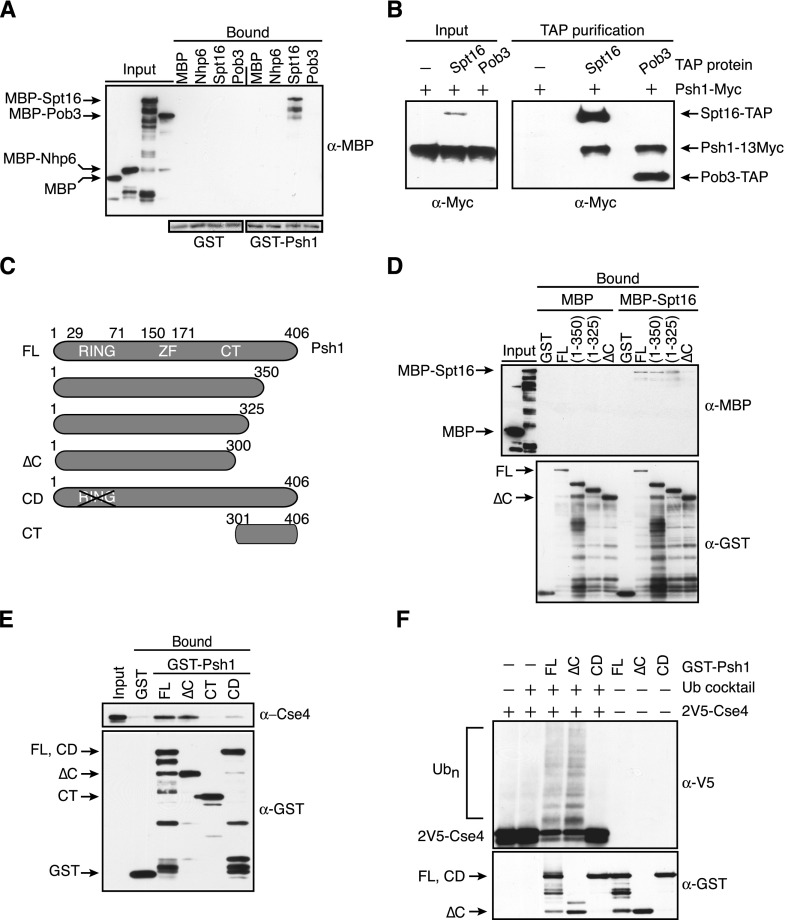
Figure 2.
Psh1 interacts with the Spt16 subunit of the FACT complex. (A) Glutathione–Sepharose resin bound to GST or GST-Psh1 was incubated with MBP or the indicated MBP fusion proteins. The Sepharose was washed and eluted, and the presence of the MBP-tagged proteins was analyzed with α-MBP antibodies. (Bottom) The blot was stained with Ponceau S to reveal GST and GST-Psh1 levels. (B) IgG Sepharose beads were used to affinity-purify Spt16-TAP (SBY9977) or Pob3-TAP (SBY9978) from cells expressing a 13Myc epitope fusion to Psh1. Cells expressing full-length Psh1-13Myc but no TAP-tagged protein (SBY6423) served as a control. The samples were subsequently analyzed by immunoblotting with α-Myc antibodies. The TAP tag contained a calmodulin-binding peptide, a TEV cleavage site, and two IgG-binding domains of protein A, the latter of which interacts with the α-Myc antibodies, allowing detection of the TAP-tagged proteins along with Psh1-13Myc. (Note: Pob3-TAP is detected in the input on longer exposures that are not shown here.) (C) Schematic of Psh1 and the fragments used in D–F. (FL) Full-length; (∆C) C-terminal deletion; (CD) catalytically dead; (CT) C-terminal region. (D) Immobilized GST alone, GST-fused full-length Psh1 [FL (1–406)], or the indicated Psh1 fragments were incubated with MBP or MBP-Spt16. The resin was washed and eluted, and the levels of the MBP and GST proteins were analyzed with α-MBP and α-GST antibodies. (E) 2V5-Cse4 octamers were added to immobilized GST alone or GST-fused full-length Psh1 (1–406), Psh1 (1–300), Psh1 (301–406), and a Psh1 RING domain mutant (C45S, C50S), followed by washing and elution from the resin. The levels of the GST proteins were assessed with α-GST antibodies, and the presence of Cse4 was assessed with α-Cse4 antibodies. (F) The indicated GST-Psh1 proteins and 2V5-Cse4 octamers were added to reactions in the presence (+) or absence (−) of a ubiquitin (Ub) cocktail. The top immunoblot shows Cse4 ubiquitylated species, while Psh1 levels are shown in the bottom immunoblot. The blots were not probed with α−ubiquitin antibodies, since we cannot distinguish Cse4 ubiquitylation from Psh1 autoubiquitylation. Furthermore, Psh1 autoubiquitylation makes the comparison of Psh1 levels in the reactions difficult, so parallel reactions were performed in the absence of the ubiquitin cocktail and 2V5-Cse4 octamers (shown in the three right lanes).