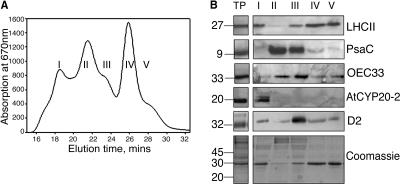
Figure 2.
A, Absorbance at 670 nm of fractions obtained from thylakoid membranes purified from LL-grown Columbia-0 plants. B, Western analysis of fractions enriched for (1) PSII supercomplexes, (2) PSI, (3) PSI + PSII core, (4) trimeric LHCII, and (5) monomeric LHCII (protein equivalent of 2 μg of chlorophyll loaded in each well). TP, total thylakoid preparation. Thylakoids were prepared by homogenizing fresh tissue in ice-cold grinding medium (330 mm sorbitol, 5 mm MgCl2, 10 mm Na4P2O7 (pH 6.5), 2 mm ascorbate) with a polytron. The homogenate was then filtered through four layers of muslin followed by two layers of muslin and one layer of cotton wool. The filtrate was centrifuged for 10 min at 5,000g, and the chloroplast enriched pellet resuspended in wash buffer (330 mm sorbitol, 10 mm MES, pH 6.5), followed by a further 10 min centrifugation at 5,000g. The pellet was then resuspended in 5 mm MgCl2 for 30 s to lyse any remaining intact chloroplasts, followed by an equal volume of osmoticum (660 mm sorbitol, 20 mm KCl, 2 mm EDTA and 100 mm HEPES, pH 6.5) to break open the stacked grana. Thylakoid membranes were freshly prepared from 4- to 5-week old Arabidopsis plants as described above with a final resuspension in 20 mm Bis-Tris (pH 6.5), 5 mm MgCl2. Thylakoid samples were diluted to a final chlorophyll concentration of 1.4 mg mL−1 chlorophyll and solubilized by addition of n-dodecyl α-d-maltoside to a final concentration of 0.7% to 0.85%. Samples were vortexed thoroughly for 1 min and centrifuged for 1 min at 16,000g. The supernatant was then filtered through a 0.45-μm nylon filter and subjected to gel filtration chromatography using a Superdex 200 HR 10/30 column in an Amersham-Pharmacia ÄKTApurifier system using the same buffer and similar conditions as before (Yakushevska et al., 2001; Ruban et al., 2003).