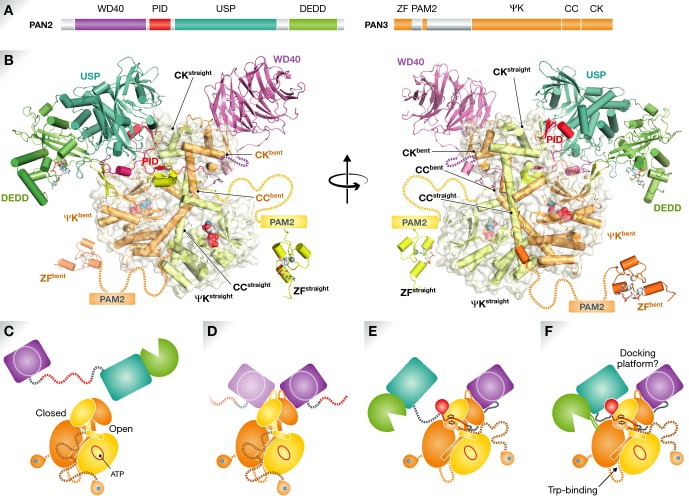
Figure 1. Organization of the Poly(A) nuclease Pan.
(A) Schematic of Pan2 and Pan3 domain (USP, ubiquitin-specific protease; DEDD, exonuclease; PID, Pan3-interacting domain; ZF, zinc finger; PAM2, PABP-interacting motif-2; ψK, pseudokinase; CC, coiled coil; CK, C-terminal knob). (B) Composite model of the Pan2–Pan3 complex derived from the available structural data (Jonas et al, 2014; Schäfer et al, 2014; Wolf et al, 2014) in two orientations. Asymmetry within the homodimeric Pan3 ψK–CK domain is most obvious in the coiled-coil helices (straight versus bent) of each protomer. The Pan2 linker connecting the WD40 and USP domains wraps around the waist of the Pan3 ψK–CK dimer, with the PID folding into the narrow groove between the ψKbent and CKstraight domains. PID and USP binding precludes recruitment of a second WD40 domain to CKstraight, ensuring 1:2 stoichiometry. The DEDD active site (marked by a docked A3 trinucleotide, sticks) is brought into vicinity of the two ψK ATP binding sites (spheres). Pan3 N-terminal zinc finger domains are presumably flexibly linked to the complex; the linker region includes PAM2. Note that it is not certain whether all interactions depicted in this composite model occur simultaneously. (C–F) Hypothetical Pan (sub)assemblies; an ordered assembly of the complex should not be inferred. (C) Both Pan2 and Pan3 possess globular N- and C-terminal domains that are thought to be separated by flexible linker peptides in the isolated proteins. (D) The asymmetric Pan3 C-terminal ψK–CK dimer is able to bind to two Pan2 WD40 domains in the absence of PID (binding), causing ordering of a conserved CK loop. (E) Engagement and folding of PID in the ψK-CK closed groove is mutually incompatible with the presence of a second Pan2 molecule. PID binding results in a restructuring of the Pan3 N-terminal segment with formation of a short antiparallel β-sheet. (F) Docking of the Pan2 USP and DEDD domains positions the nuclease active site close to the ATP binding sites of ψK, which binds to and structures an otherwise disordered DEDD domain loop. See text for further details.