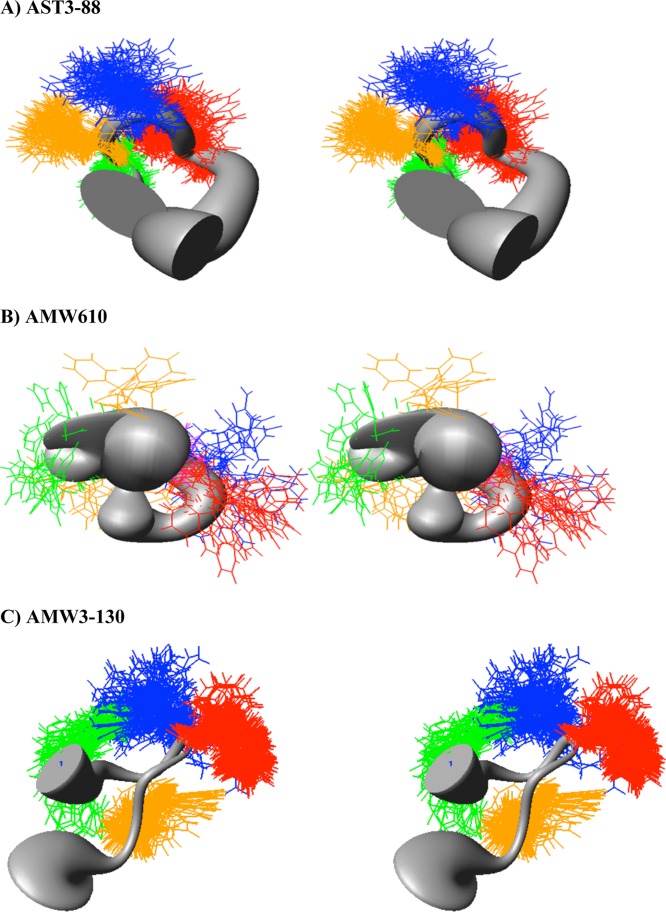
Figure 4.
Sausage stereoview representations of the major family members of compound (A) AST3-88 (in the H, F, R,W region; RMSD = 1.16 ± 0.45 Å), (B) AMW610, and (C) AMW3-130 (in the H, F, R,W region; RMSD = 0.66 ± 0.3 Å) aligned on the backbone heavy atoms of residues 3–7. The His side chain is indicated in green, the DPhe side chain in yellow, the Arg side chain in blue, and the Trp side chain in orange. A wide gray backbone indicates greater flexibility in that domain. Following restrained molecular dynamics (RMD) simulations for 10 ns, 200 equally spaced structures were energy minimized with the NMR based restraints. The energy minimized structures were grouped into conformational families by comparison of the backbone dihedral angels within the His-Phe-Arg-Trp domain.