Figure 6. Modulation of protein interactions by phosphomimetic mutations in VGLUT1.
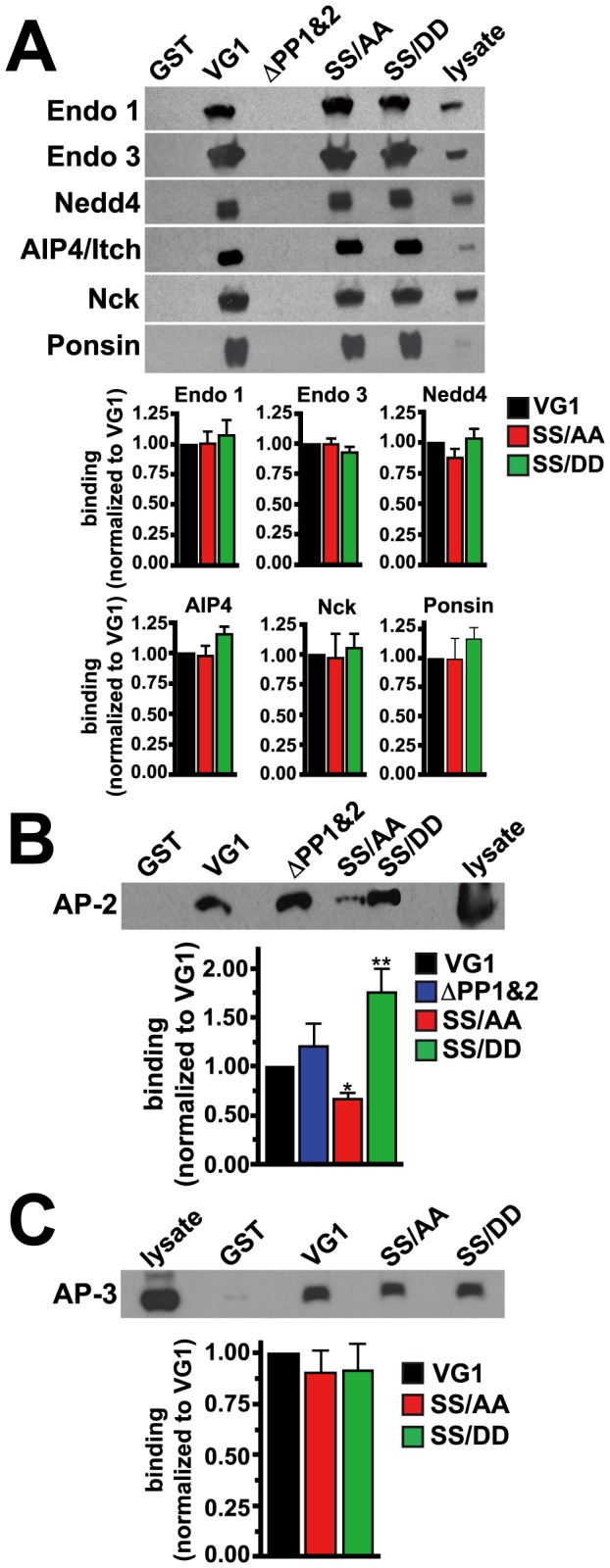
GST pull-down assays were performed by incubating rat brain extracts with GST, or GST fusions of the WT VGLUT1 C-terminus (VG1), or mutants deleting both polyproline domains (ΔPP1&2), or mimicking the unphosphorylated (SS/AA) or phosphorylated state (SS/DD). (A) Bound proteins were detected by immunoblotting with antibodies against endophilin 1, endophilin 3, Nedd4, AIP4/Itch, Nck, and ponsin. Phosphomimetic mutations did not affect binding compared to VG1. Deletion of the polyproline motifs (ΔPP1&2) prevents binding of the polyproline domain interacting proteins. (B) Bound proteins were detected by immunoblotting with antibody against AP-2. The phosphomimetic SS/DD mutation promotes increased binding of VGLUT1 to AP-2 (1.761±0.2422 a.u.), while SS/AA mutation decreases binding of VGLUT1 to AP-2 (0.6745±0.0668 a.u.). (C) Bound proteins were detected by immunoblotting with antibody against AP-3. Binding of VGLUT1 to AP-3 is unaffected by serine mutations. Top panels show representative immunoblots, bottom panels show the averaged quantification of band intensity from at least three independent experiments. *p<0.05, **p<0.01, one-way ANOVA with Bonferroni's post-test.
