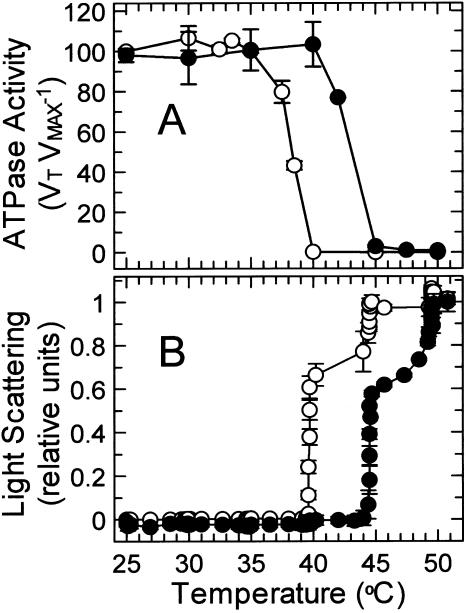
Figure 8.
Effect of temperature on the stability of recombinant activase from Antarctic hairgrass and creosote bush. A, Residual ATPase activity of recombinant activase from Antarctic hairgrass (○) and creosote bush (•) was measured at 30°C after incubation for 10 min at the indicated temperatures in the presence of 0.75 mm ATPγS. Results are expressed as VT VMAX−1, the ratio of the activities after incubation at the indicated temperature (VT) to the control activity (VMAX) determined for enzyme maintained at 4°C. The rates of ATP hydrolysis by the controls were 0.85 and 0.51 units mg protein−1 for activase from Antarctic hairgrass and creosote bush, respectively. B, Thermal aggregation of recombinant activase from Antarctic hairgrass (○) and creosote bush (•) was determined by measuring light scattering at the indicated temperatures during a time course of increasing temperature. Recombinant activase (40 μg) was incubated in 400 μL in a thermostatted cuvette in the presence of 0.75 mm ATPγS. After 5 min at 25°C, the temperature of the cuvette was increased by increasing the temperature of circulating water bath by 5°C every 10 min.