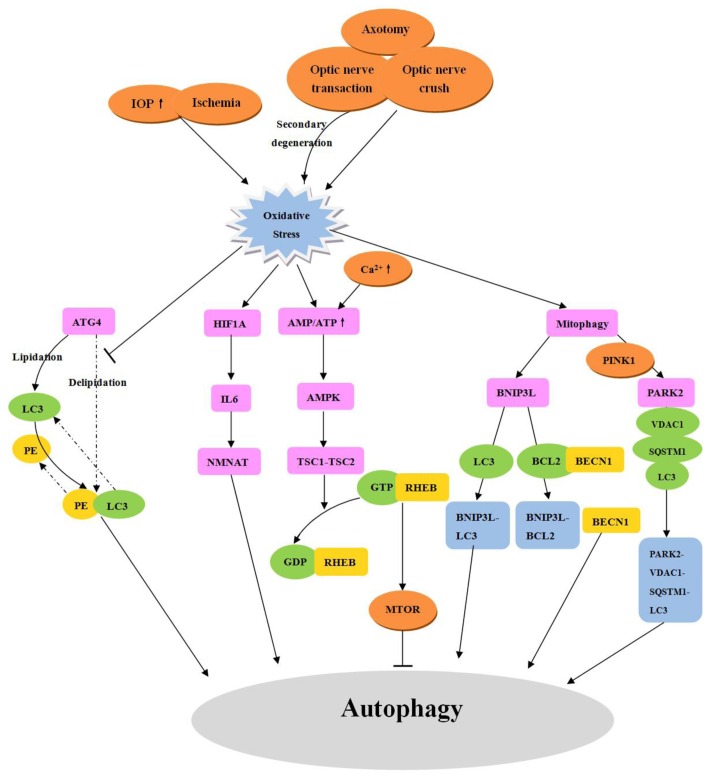
Figure 3. Regulation of autophagy by oxidative stress in RGCs. Various injuries cause oxidative stress in RGCs by primary and secondary degeneration, and thereby induce autophagy. ATG4 has 2 effects: A) it primes ATG8 homologs for conjugation with PE, allowing for lipidated LC3 and GABARAP family protein incorporation into the phagophore membrane, and B) deconjugation of LC3 and GABARAP protein from phosphatidylethanolamine apparently permits recycling of these proteins. Increased concentrations of ROS inactivate the second function of ATG4. In response to oxidative stress, HIF-1 is activated, which indirectly augments NMNAT via induction of IL6. Additionally, NMNAT may act on the upstream pathways of autophagy. Oxidative stress and increasing intracellular Ca2+ levels will activate AMPK, which promotes the formation of a TSC1-TSC2 complex, leading to MTOR inactivation and initiation of autophagy. Under oxidative stress, mitophagy is activated. BNIP3L localizes to the mitochondrial outer membrane, it interacts directly with LC3 or GABARAP, and mediates the recruitment of damaged mitochondria to phagophores. BNIP3L also competes with BECN1 for binding to BCL2 and thereby releases BECN1 to induce autophagy. PINK1 recruits the E3 ligase PARK2 to damaged mitochondria, which then mediates mitochondrial fragmentation and targeting to phagophores, which is followed by ubiquitination of VDAC1 and binding to SQSTM1. Then SQSTM1 targets this complex to the autophagosome by interacting with the autophagic protein LC3.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.