Abstract
Objective
Microalbuminuria (MA), a marker of renal microvascular disease, is associated with brain atrophy and neurovascular changes in older adults with type 2 diabetes mellitus (DM). We evaluated the relationship between urine albumin-to-creatinine ratio (UACR) and regional brain volumes determine whether subclinical albuminuria may indicate early structural brain changes in type 2 DM.
Materials/Methods
We studied UACR and brain volumes in 85 type 2 DM patients (64.8 ± 8.3 years) and 40 age-matched controls using 3D magnetization prepared rapid acquisition with gradient echo (MP-RAGE) MRI (magnetic resonance imaging) at 3 Tesla. The relationship between UACR and brain volumes was analyzed using the least square models.
Results
In DM patients, UACR ≥ 5 mg/g, UACR ≥ 10 mg/g and clinically significant MA (UACR ≥ 17 mg/g [males] and 25 mg/g [females]) were associated with lower gray matter (GM) volume in the frontal lobe (r2 adj = 0.2-0.4, P= 0.01-0.05) and UACR ≥ 5mg/g was also related to global GM atrophy (r2 adj= 0.1, P= 0.04), independent of DM duration, glucose levels, HbA1c and hypertension. For UACR ≥ 5mg/g, a lower global GM volume was related to worse executive function (P= 0.04) in the DM group. No associations were found for UACR (< 5 mg/g) and controls.
Conclusions
Subclinical albuminuria (UACR ≥ 5 mg/g) is associated with lower GM volume that has clinical impact on cognitive function in older diabetic patients, and these relationships are independent of DM control and hypertension. Therefore, UACR levels may serve as an additional marker of DM-related brain structural changes.
Keywords: Microvascular Disease, Microalbuminuria, Brain Atrophy, Diabetes Complications
1. Introduction
Type 2 diabetes mellitus (DM) is associated with endothelial dysfunction affecting the kidney and the brain, but the link between albuminuria and brain tissue damage remains unclear. Endothelial sensitivity to and the time course of vascular changes may differ between the central and the peripheral circulation [1-6].
Albuminuria, a marker of chronic vascular kidney disease, is associated with accelerated cognitive decline in diabetic patients [2]. Cerebromicrovascular disease manifests as impaired vasoreactivity [7, 8], hypoperfusion and decreased metabolism that may lead to hypoxia and brain tissue loss [9-12]. Therefore, cognitive and motor regions such as the frontal and parietal lobes are particularly vulnerable to hyperglycemia because of their high metabolic rates [13, 15]. Older diabetic patients have lower brain volumes, larger ventricular volumes, [9] and faster progression of brain atrophy [16, 17].
The relationships among brain atrophy, hyperglycemia and DM duration have been widely studied [1, 5, 6, 18-20], but the link between albuminuria and structural brain changes has not been well characterized. Therefore, as a systemic vascular dysfunction indicator, albuminuria may be associated with type 2 DM complications in the brain that may be prodromal for cognitive impairment later in life.
We hypothesized that: 1) Albuminuria is associated with gray matter (GM) loss in older people with type 2 DM. Subclinical levels of urine albumin-to-creatinine ratio (UACR) (UACR ≥ 17 mg/g [males], 25 mg/g [females]) may indicate brain structural changes. 2) The relationship between UACR and brain structure is independent of DM control and duration. We aimed to determine the association between UACR and brain structural changes in type 2 DM and control patients.
2 Materials and Methods
2.1 Participants
This study was conducted at the Syncope and Falls in the Elderly Laboratory at the Beth Israel Deaconess Medical Center and at the Center for Advanced Magnetic Resonance Imaging, Department of Radiology.
We reviewed records obtained from January 2002 to July 2012, collected during three studies: 1) Cerebral vasoregulation in diabetes (January 2002-December 2005); 2) Cerebral perfusion and cognitive decline in type 2 diabetes (January 2006-December 2009); and 3) Cerebromicrovascular disease in elderly with diabetes (August 2009-July 2012) (see funding section for details).
Participants were recruited from the local community via advertisements. All subjects signed an informed consent approved by the Institutional Review Board. Inclusion criteria for the diabetes group (men and women diagnosed with type 2 DM > 1 year, normotensive or hypertensive, age > 50 years old) were similar among these studies. For the control group, however, studies 1 and 2 included only normotensive non-diabetic participants, while study 3 included both normotensive and hypertensive participants.
A total of 308 participants signed informed consent (120 non-diabetics, 188 diabetics). One hundred twenty two participants (52 non-diabetics, 70 diabetics) were excluded for one of the following reasons: type 1 DM, stroke or recent myocardial infarction, clinically relevant cardiac disease (arrhythmias, kidney or liver transplant, carotid artery stenosis), neurologic disorder, other systemic disorders, uncontrolled HTN or treatment with 3 or more antihypertensive agents, claustrophobia, metal implants, arterial stents and pacemakers not compatible with 3Tesla MRI, no transcranial Doppler window, withdrew consent, lost to follow-up or other reasons. For this study, we also excluded 61 records with incomplete datasets (missing UACR or GM volumetric measurements). Forty three patients that were excluded had HTN diagnosis. We analyzed data from 125 subjects.
2.2 Study Cohort
The DM group consisted of 85 men and women, aged 50-85 years, who were diagnosed with type 2 DM and treated with oral agents and/or combinations with insulin; they were normotensive (blood pressure [BP] < 140/90 mmHg and had no medical history of hypertension [HTN]) or hypertensive (n= 51) (BP ≥ 140/90 mmHg and/or treated for HTN). Subjects were treated with one or more of the following: insulin (6), oral glucose-control agents (sulfonylurea, second generation agents or a combination) (56), both (15) and/or diet (8). The control group consisted of 40 age-matched (± 5 years) men and women; seven of them treated for HTN. The control group had normal fasting blood glucose and HbA1c levels. They also had similar cardiovascular risk factor distribution (HTN, hypercholesterolemia, obesity, history of cardiovascular disease) as the type 2 DM group.
2.3 Study Protocol
All participants were screened using medical history, autonomic symptoms and activity questionnaires, ECG, vital signs, height, weight and body mass index (BMI). Blood was drawn to measure fasting blood glucose, HbA1c, renal panel, lipid profile and complete blood count. Neuropsychological assessments included executive function (Trail Making Tests A and B (TMT); verbal learning and memory (Hopkins Verbal Learning Test) tests, as well as the Instrumental Activities of Daily Living scale. Systolic, diastolic and mean BP measurements were obtained every 20 minutes using a wearable 24-hour home monitoring device (Dynapulse, Inc. Vista, CA).
2.4 Urine albumin creatinine ratio and microalbuminuria
UACR was measured from the first-void clean-catch urine samples collected on the day participants were scheduled for brain MRI. Urinary albumin was measured by immunoassay and urinary creatinine by the modified Jaffe method.
Microalbuminuria (MA) was defined as an UACR ≥ 17- 250 mg/g in men and 25-350 mg/g in women per the sex specific in spot urine sample [21]. These sex-specific cutoffs, when converted to albumin excretion rates, became almost equal at 30 mcg/min [1, 5, 18-20]. We adopted the UACR ≥5 mg/g and UACR of ≥10 mg/g as cut-off ranges for the analyses, based on epidemiological studies that showed their association with an increased risk for HTN and cardiovascular events [22, 24].
2.5 Magnetic Resonance Imaging
Studies were performed on a 3-Tesla GE GHX MRI scanner using a quadrature and eight-channel phase array head coils (GE Medical Systems, Milwaukee, WI). Anatomical images were acquired using 3-D magnetization prepared rapid acquisition with gradient echo (MP-RAGE) and fluid attenuated inversion recovery (FLAIR) sequences. Images were analyzed using tools developed in interactive data language (IDL, Research Systems, Boulder, Colorado, USA) and MATLAB (MathWorks, Natick, Massachusetts, USA). Anatomical magnetic resonance images (MP-RAGE and FLAIR) were co-registered non-linearly to the MNI152 standard template and segmented to calculate regional GM, white matter (WM) and cerebrospinal fluid (CSF) volumes in main anatomical lobes and their subregions (SPM, University College London, UK) [25]. MP-RAGE and FLAIR images were co-registered to a standard template and segmented to calculate regional GM, WM, CSF and white matter hyperintensities (WMHs) volume in the frontal, temporal, parietal and occipital regions using statistical parametric mapping software package (SPM, University College London, UK). WMHs were identified by thresholding of hyperintense pixels on FLAIR images with > 30% increase in signal intensity compared with the global WM average for each patient, and normalized for total brain volume [26].
2.6 Imaging Analysis
All the images were processed on a Linux workstation using tools developed in the IDL programming environment (Research systems, Boulder, CO) [10]. Three dimensional regions of interest corresponding to parenchymal brain were extracted using the “brain extraction tools” algorithm [27] on the MP-RAGE and FLAIR images to segment gray and WM and CSF. Subregions were defined according to the LONI Probabilistic Brain Atlas (LPBA40). Each lobe (e.g. frontal, temporal etc.) was divided according to anatomical divisions and structures (e.g. middle orbitofrontal gyrus, superior frontal gyrus, hippocampus, etc.).
2.7 Statistical Analysis
The JMP software 11 PRO (SAS Institute, Cary, NC) was used for analyses. Descriptive analyses were used for demographic characteristics and one-way ANOVA and Wilcoxon tests for group comparisons between type 2 DM and control groups. Linear regression and least square (LS) models were used to find relationships between albuminuria, brain volumes and WMHs. Global CSF volume was included in all the analyses as co-variant to account for differences in regional atrophy and head size [28].
UACR ≥ 5 mg/g; UACR ≥ 10mg/g and MA [≥ 17mg/g (M), ≥ 25 mg/g (F)] were used as independent variables. The dependent variables were global and regional GM and WM volume and WMHs. The relationships between UACR, MA and brain volumes were analyzed using unadjusted linear regression and four least square models (LS) adjusted for (A-CSF; B-CSF, age and gender; C-CSF, HTN years and glucose levels; D- CSF, diabetes duration and HbA1c). We present r2 adjusted for model covariates and we conservatively selected models with adjusted r2 adj > 0.1, and P < 0.05. Each brain region was modeled separately to minimize repeated measures effects. The effect of smoking, alcohol abuse and other confounders was also evaluated.
3. Results
3.1 Characteristics of the Study Cohort
The characteristics of the study cohort are listed in Table 1. The type 2 DM group had higher BMI, HbA1c, glucose, 24 hour systolic BP, longer HTN duration, worse cognitive performance, lower GM and WM volumes, as well as a slower preferred walking speed. Other characteristics, such as smoking pack years, C-reactive protein, urine creatinine, urine albumin and WMHs volume were similar between the groups. Control participants had higher cholesterol blood levels due to untreated hypercholesterolemia (11 participants).
Table 1. Demographic and clinical characteristics by group.
| Characteristics | Diabetes n=85 (mean, STD deviation) | Control n=40 (mean, STD deviation) | P value |
|---|---|---|---|
| Age (years) | 64.8 ± 8.3 | 67.5 ± 9.9 | 0.1 |
| Males/Females | 47/38 | 20/20 | NS |
| BMI (kg/m2) | 28.4 ± 6.5 | 24.5 ± 5.1 | 0.001 |
| Hypertension | 51 (60.0%) | 7 (17.5%) | < 0.0001 |
| Hypertension (years) | 6.7 ± 9.1 | 2.07 ± 5.01 | 0.003 |
| Diabetes duration (years) | 11.6 ± 9.5 | NA | NA |
| 24hr systolic blood pressure (mmHg) | 133.6 ± 7.9 | 128.3 ± 7.6 | 0.01 |
| 24hr diastolic blood pressure (mmHg) | 70.9 ± 7.3 | 68.2 ± 8.0 | 0.1 |
| Alcohol intake dose/week | 4.0 ± 12.8 | 1.39 ± 2.9 | 0.2 |
| Smoking pack (years) | 14.4 ± 19.8 | 18.6 ± 19.4 | 0.3 |
| Hemoglobin (g/dl) | 13.2 ± 1.3 | 13.4 ± 1.3 | 0.4 |
| HbA1c (%) (mmol/mol) | 7.2 ± 1.2 | 5.6 ± 0.3 | < 0.0001 |
| Fasting Glucose (mg/dl) | 120.5 ± 45.5 | 86.2 ± 11.9 | < 0.0001 |
| Urine creatinine (mg/dl) | 103.4 ± 59.5 | 90.7 ± 43.7 | 0.2 |
| Albumin creatinine ratio (mg/g) | 15.4 ± 27.4 | 23.9 ± 47.3 | 0.2 |
| Microalbuminuria | 70.5 ± 53.7 | 116.3 ± 71.7 | 0.1 |
| (30-300 mg/g) | n= 10 (8%) | n= 6 (4.8%) | |
| UACR ≥ 17 mg/g (M), ≥ 25 mg/g (F) | 49.4 ± 46 | 103.7 ± 73.5 | 0.03 |
| n= 18 (14.4%) | n= 7(5.6%) | ||
| UACR ≥ 10 mg/g | 33.6 ± 39.4 | 56.9 ± 66.1 | 0.1 |
| n= 31 (24.6%) | n= 15 (11.9%) | ||
| UACR ≥ 5 mg/g | 21.4 ± 31.9 | 40.8 ± 59.1 | 0.06 |
| n= 57 (45.6%) | n= 22 (17.6%) | ||
| UACR < 5 mg/g | 3.2 ± 0.8 | 3.2 ± 1.19 | 0.9 |
| n= 29 (23.2%) | n= 18 (14.4%) | ||
| C-reactive protein (mg/L) | 2.4 ± 4.2 | 1.4 ± 1.7 | 0.1 |
| Cholesterol (mg/dl) | 170.2 ± 41.3 | 191.4 ± 46.9 | 0.01 |
| Retinopathy grading (0-9) | 0.6 ± 1.7 | 0 | NA |
| Global intracranial volume (ml)a | 1555.8 ± 240.8 | 1637.7 ± 240 | 0.02 |
| Frontal lobe volume gray matter volume (ml)a | 97.9 ± 1.1 | 100.5 ± 1.7 | 0.2 |
| Global gray matter volume (ml)a | 623.46 ± 64.6 | 651.1 ± 81.5 | 0.01 |
| Global white matter (ml)a | 422.3 ± 58.1 | 436.7 ± 57.7 | 0.1 |
| Global WMHs (ml)a | 13.1 ± 9.7 | 12.4 ± 7.1 | 0.6 |
| Global CSF volume (ml)b | 510.2 ± 162.4 | 549.9 ± 185.4 | 0.1 |
| Gait Speed (m/s) | 1.05 ± 0.1 | 1.13 ± 0.1 | 0.01 |
| TMT: T score | 47.0 ± 11.6 | 49.1 ± 10.1 | 0.3 |
| HVLT Total Recall T-score | 45.6 ± 12 | 54.4 ± 11.1 | 0.0004 |
| HVLT Delayed Recall T-score | 41.8 ± 13.2 | 50.6 ± 11 | 0.001 |
Volumes were compared using least squares models adjusted for CSF, age, gender and group (reported parameters: LS mean and STD error).
Volumes were compared using least squares models adjusted for age, gender and group (reported parameters: LS mean and STD error).
Abbreviations: standard (STD); Least squares (LS); Urine albumin creatinine ratio (UACR); Males (M); Females (F); White matter hyperintensities (WMHs); Cerebrospinal fluid (CSF); Hopkins verbal learning test (HVLT); Instrumental activities of daily living scale (IADL); Trail making test (TMT).
3.2 Relationship between UACR and MA with Brain Structural Changes
In diabetic participants, increasing UACR levels were associated with global and regional GM atrophy within the frontal and occipital lobes. Figure 1 demonstrates this relationship between UACR ≥ 5 mg/g and global and frontal lobe GM in an adjusted LS models for age and gender in type 2 DM patients. UACR < 5 mg/g was not associated with brain volumes or functional measures.
Figure 1.
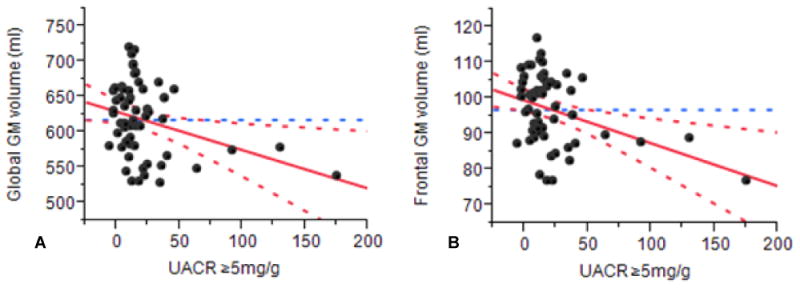
Association of UACR ≥ 5 mg/g with lower global (A) (r2adj= 0.3, P= 0.01) and regional (B) (frontal lobe: r2 adj= 0.3, P= 0.005) gray matter volume in diabetic patients independent of global cerebrospinal fluid volume (ml), age and gender. Abbreviations: GM (gray matter); UACR (urine albumin creatinine ratio).
Table 2 shows functional associations among GM volumes and UACR ≥ 5 mg/g using a linear regression (unadjusted) and four models (A-D) adjusted for age, gender, HTN years, glucose levels, DM duration, HbA1c and CSF volume. Within the DM group, UACR levels ≥ 5 mg/g were associated with global (r2 adj= 0.1, P= 0.04) GM atrophy. Within the frontal lobe, increasing UACR levels were associated with regional GM atrophy (UACR ≥ 5 mg/g [r2 adj= 0.2, P= 0.01]; UACR ≥ 10 mg/g [r2 adj = 0.3, P= 0.05]). In the frontal lobe GM, the strongest association with UACR ≥ 5 mg/g (r2 adj= 0.1, P= 0.009) was found in model C adjusted for CSF, HTN years and glucose levels. These relationships were also significant for models A, B and D. UACR levels ≥10 mg/g were also associated with lower frontal lobe GM in all models (e.g. model B, r2 adj= 0.4, P= 0.01) and lower global GM volume in model B (r2 adj = 0.4, P=0.03). UACR levels ≥ 17 mg/g (men) and ≥ 25 mg/g (women) were associated with lower global (r2 adj= 0.3, P= 0.01-0.02) and regional frontal lobe (r2 adj= 0.4, P= 0.01) GM volumes on models C and D. We also tested log UACR, and detected similar associations with GM volume. UACR < 5 mg/g was not associated with GM changes. UACR was not associated with WM volume or WMHs in the DM group. In controls, UACR was not associated with GM changes.
Table 2. Relationship between UACR and GM volumes (type 2 DM group).
| UACR ≥ 5 mg/g | Global GM | Global frontal lobe | Superior frontal gyrusa | Middle frontal gyrusa | Inferior frontal gyrusa | Middle orbitofrontal gyrusa | Lingual gyrusa | |
|---|---|---|---|---|---|---|---|---|
| Linear | r2 adj | 0.07 | 0.1 | 0.08 | 0.1 | 0.08 | 0.1 | 0.07 |
| Regression | P | 0.02 | 0.006 | 0.01 | 0.003 | 0.01 | 0.008 | 0.02 |
| LS Model–A | r2 adj | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0.4 | 0.3 |
| P | 0.04 | 0.01 | 0.02 | 0.006 | 0.02 | 0.01 | 0.03 | |
| LS Model–B | r2 adj | 0.3 | 0.3 | 0.3 | 0.2 | 0.1 | 0.3 | 0.4 |
| P | 0.01 | 0.005 | 0.008 | 0.01 | 0.02 | 0.01 | 0.01 | |
| LS Model–C | r2 adj | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.3 |
| P | 0.03 | 0.009 | 0.01 | 0.01 | 0.02 | 0.01 | 0.05 | |
| LS Model–D | r2 adj | NS | 0.2 | 0.1 | 0.2 | 0.1 | NS | 0.4 |
| P | NS | 0.02 | 0.05 | 0.02 | 0.04 | NS | 0.02 |
Left hemisphere
Model A= adjusted for CSF only; Model B= adjusted for CSF, age and gender; Model C= adjusted for CSF, hypertension years and glucose levels; Model D= adjusted for CSF, diabetes duration and HbA1c. Abbreviations: Urine albumin creatinine ratio (UACR); Gray matter (GM); Diabetes mellitus (DM); Least squares (LS); Cerebrospinal fluid (CSF).
Figure 2 shows the GM volume for each UACR range for the DM and the control groups (Figure 2A) and for the entire cohort (Figure 2B). Ten DM participants with clinically significant MA (UACR 30-300 mg/g) had lower global GM volume as compared to diabetic subjects with UACR < 5 mg/g by 8.5% (p=0.03). In the DM group, as UACR increased, GM volume decreased by at least 30 cm as compared to controls (UACR ≥ 5 mg/g [r2 adj= 0.3, P= 0.04]; 612.08 cm3 [type 2 DM], 649.929 cm3 [controls]). DM subjects with MA, had also lower frontal lobe GM volume (r2 adj=0.4, P=0.04), and lower orbitofrontal gyrus GM volume bilaterally (r2 adj= 0.8, P= 0.02-0.03) (model adjusted for CSF, HTN and glucose levels) as compared to controls. For the entire cohort (Figure 2B), GM volume was lower for subjects with UACR ≥ 5 mg/g (p=0.03), UACR ≥10 mg/g (p=0.02) and MA (p=0.03) as compared to subjects with an UACR closer to normal. Urine creatinine ≥ 1.2g) was not associated with structural brain changes.
Figure 2.
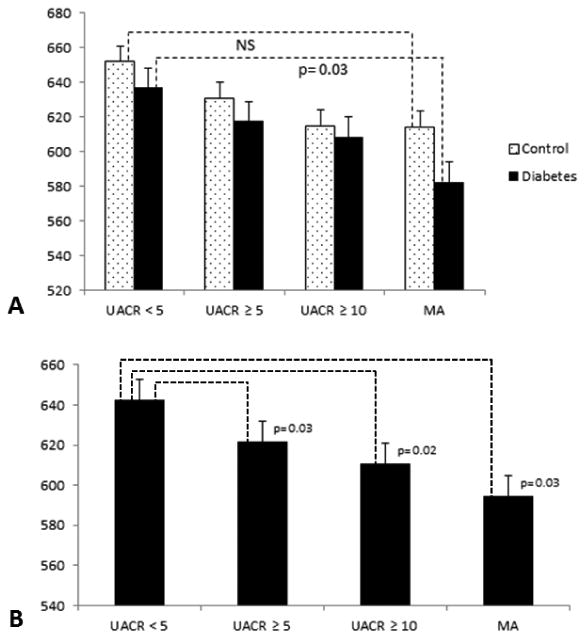
A. Mean global gray matter (GM) volume for each urine albumin-to-creatinine ratio (UACR [mg/dl]) subgroup for the diabetes (dotted bars) and the control (black bars) groups. GM volume was lower in the diabetic participants with MA as compared to diabetic participants with UACR<5 [mg/dl]. B. For the entire cohort, GM volume was lower for participants with UACR ≥5 (p=0.03), UACR >10 (p=0.02) and MA (p=0.03) as compared to participants with an UACR closer to normal.NJ
3.3 Relationship between gray matter atrophy, cognition and diabetes control
The type 2 DM group performed worse than controls on cognitive measures of learning and memory (Table 1, HVLT total recall [P= 0.0004], HVLT delayed recall [P= 0.001]). In DM subjects, lower GM volumes were associated with worse executive function performance on TMT T scores (A and B). This relationship was observed for global GM, superior frontal, right middle frontal, right lingual and middle orbitofrontal GM gyri (r2 adj= 0.05-0.2, P= 0.006-0.03). DM severity (i.e. glucose levels, HbA1c and disease duration) was not associated with global and frontal lobe GM atrophy.
3.4 Relationship between gray matter atrophy and hypertension
In diabetic patients, lower global (r2 adj= 0.2, P= 0.02) and frontal (r2 adj= 0.2, P=0.03) GM volumes were associated with higher UACR ≥ 5 mg/g independent of global CSF volume, HTN diagnosis, and 24h systolic BP. A systolic BP effect on global GM (P= 0.02) and frontal lobe GM (P= 0.04) was observed, but systolic BP was not associated with UACR. In controls, systolic BP correlated with higher UACR ≥ 5 mg/g (r2 adj= 0.09, P= 0.01) but not with GM volumes. Models including the effect of diastolic BP and mean BP were not significant. The volume of global WMHs was not associated with higher UACR in patients with DM, HTN or both.
3.5 Relationship between gray matter atrophy and BMI
BMI was not associated with global gray matter volume in the diabetic group or in the entire cohort (linear regression, LS models). However, higher BMI was associated with slower gait speed in the control group (r2= 0.09, p= 0.0002) and in the entire cohort (r2= 0.04, p=0.0007), but not in the DM group. BMI showed no correlation with 24-hour systolic blood pressure.
4. Discussion
This study reports that UACR ≥ 5 mg/g is associated with lower GM volume in type 2 DM. UACR-GM relationships were observed for global GM measures and regionally (frontal lobe), and they were independent from DM duration, fasting glucose levels, HbA1c and HTN. The UACR-GM associations were detected even at a UACR ≥ 5 mg/g, which is lower than clinical MA threshold. Diabetic subjects with MA had 8.5% lower GM volume as compared to those with normal UACR. The degree of GM atrophy was clinically relevant and its manifestation was evident as a worse cognitive function in diabetic participants. In particular, we found a correlation between UACR, frontal lobe atrophy and executive dysfunction. This relationship is clinically relevant because DM imposes a risk for cognitive decline and dementia. A urine creatinine value ≥ 1.2g) was not linked to GM atrophy, demonstrating a stronger relationship between UACR and brain volume changes. UACR-GM associations were not detected in the control group, even though they had similar UACR levels and risk factors (e.g. HTN, hyperlipidemia). Therefore, these findings suggest that the adverse effects of endothelial dysfunction associated to chronic hyperglycemia may impact both the kidney and the brain even before MA is clinically diagnosed.
MA associated to endothelial dysfunction in diabetic nephropathy causes diffuse microvascular changes affecting the efficacy of glomerular filtration [29, 30]. Similarly, DM-related cerebral microvascular disease is associated with lower cortical perfusion, and diffuse vascular manifestations in GM, WMHs and lacunar infarcts [31]. In our study, UACR and MA were correlated with lower GM volume, suggesting a similar microvascular pathophysiology affecting the kidney and the brain. This relationship between UACR and frontal lobe GM may be explained by the impact of DM on brain aging. In normal aging, frontal lobe GM volume begins to decline around the fifth decade of life [31, 32]; type 2 DM may accelerate this process [17, 33].
Other contributing factors to the decreased brain volume in the type 2 DM group may be reduced cerebral blood flow, vasoreactivity and metabolism [9] that may lead to structural changes within GM and neurovascular units. As it has been previously reported, we did not find significant associations between UACR and WMHs in type 2 DM participants [16, 31, 34]; our study analyzed structural changes in cerebral cortex and white matter separately. The link between MA and brain volume reduction was only observed on GM structures.
MA, as an early marker of endothelial dysfunction and early predictor of renal disease (usually precedes the decline in glomerular filtration rate), may also be a signal of early-stage microvascular brain damage [35]. Epidemiological studies show that an UACR ≥ 5 mg/g is independently correlated with incidental HTN [22, 23]. An UACR of ≥ 10 mg/g is linked with increased mortality [23]; this risk further increases for every 4 mg/g raise in UACR [24]. In diabetic participants, the negative relationship between UACR ≥ 5 and GM volume was observed independent of 24h systolic BP and WMHs, supporting the hypothesis that DM is the major contributor of microvascular disease and GM loss in this cohort. UACR was not predictive of cognitive decline, but lower gray matter volumes were associated with executive dysfunction and higher UACR; these findings suggest that UACR may potentially hallmark early GM structural changes that may over time manifest as impaired cognitive performance. In our study, the association of UACR ≥ 5 mg/g) with GM atrophy supports the notion that subclinical levels of UACR may have clinical predictive value as an early marker of brain structural changes in type 2 DM. Therefore, the adverse effect of UACR on the brain may have begun even before MA is detected.
There are potential limitations of this study. UACR was calculated from a single spot early morning urine sample; however, this method has been shown to correlate with 24-hour urine albumin excretion rates [36]. Recent data have shown that early morning urine sample is a more reliable predictor of albumin excretion as compared to a random urine sample [37]. In this study, there was a small number of DM patients with MA (n= 10, range 30-300 mg/g), and especially with severe MA (n= 5). However, global GM volume was lower in the DM participants with MA, as compared to participants with DM and normal UACR (Figure 2). This was not observed in the control group. Therefore, in our study, patients with DM and MA were more likely to show reduced GM volumes as compared to control patients with MA.
This retrospective analysis of prospectively collected data included a small number of subjects with clinically significant MA (30-300 mg/g); therefore, UACR ≥ 17 mg/g in men > 25 mg/g in women) for spot urine sex specific MA was used. The number of non-diabetic hypertensive participants was limited due to recruitment difficulties. A prospective study with larger cohort would allow to further validate the observed results and to determine whether UACR can be used as predictor of brain damage and cognitive function loss.
Frontal lobe GM atrophy correlated with cognitive decline in diabetic participants, supporting previously reported relationships between vascular disease, frontal lobe atrophy and dementia in DM [38-42]. In contrast, in non-diabetic patients, similar UACR levels were not associated with significant brain volume loss. Although our correlational study was unable to elucidate the causal link between UACR and brain atrophy [29], other studies support the notion that hyperglycemia-associated microvascular disease contributes to brain loss [43].
5. Conclusions
This study demonstrated a link between UACR and GM volume changes in type 2 DM subjects as compared to age-matched controls. Therefore, even subclinical levels of UACR ≥ 5 mg/g) may hallmark an early decline in brain health. The latter changes present as GM volume loss, which has consequences on cognitive performance in older people with type 2 DM.
Subclinical albuminuria, an inexpensive diagnostic tool, may be used as an early associative marker of brain structural changes; this requires further investigation [35, 37]. Despite the relatively young age prevalent in our cohort, participants suffered from cognitive impairment. Brain volume reduction might be an early effect of microvascular disease, which further leads to functional changes such as cognitive decline and dementia. Therefore, UACR-GM relationships may be prodromal of severe cognitive impairment in the future. UACR levels may serve as an additional marker of DM-related brain structural changes, and thus add to a repertoire of clinically relevant, inexpensive diagnostic tools that can be used in this vulnerable population to identify diabetic patients at a greater risk of functional decline.
Acknowledgments
Daniela Pimentel is also a medical student of Tecnologico de Monterrey Medical School, Mexico.
Funding: NIH–National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (5R21-DK-084463-02) and the NIH–NIA (1R01-AG-0287601-A2) (awarded to V.N.) | The Harvard Clinical and Translational Science Center (NIH Award KL2 RR 025757). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the NIH.
Abbreviations
- MP-RAGE
3-D magnetization prepared rapid acquisition with gradient echo
- r2adj
Adjusted correlation coefficient
- BP
Blood pressure
- CSF
Cerebrospinal fluid
- DM
Diabetes Mellitus
- FLAIR
Fluid attenuated inversion recovery
- GM
Gray matter
- HTN
Hypertension
- LS
Least squares
- MRI
Magnetic Resonance Imaging
- MA
Microalbuminuria
- mcg/min
Micrograms per minute
- mg/g
Milligrams per gram
- TMT
Trail making test
- UACR
Urine Albumin Creatinine Ratio
- WM
White matter
- WMHs
White matter hyperintensities
Footnotes
Conflict of Interest: The authors have no conflicts of interest and have nothing to disclose.
Author contributions: D. M. and D. P. analyzed the data, performed statistical analyses and wrote the manuscript. A.A contributed to MRI analysis. M.Z. N. contributed to manuscript preparation and data analysis. V. N. designed the study, conducted experiments, and oversaw all aspects of the study, data interpretation and manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barzilay JI, Gao P, O'Donnell M, et al. Albuminuria and decline in cognitive function: The ONTARGET/TRANSCEND studies. Arch Intern Med. 2011;171(2):142–150. doi: 10.1001/archinternmed.2010.502. [DOI] [PubMed] [Google Scholar]
- 2.De Bresser J, Reijmer YD, van den Berg E, et al. Microvascular determinants of cognitive decline and brain volume change in elderly patients with type 2 diabetes. Dement Geriatr Cogn Disord. 2010;30(5):381–386. doi: 10.1159/000321354. [DOI] [PubMed] [Google Scholar]
- 3.Den Heijer T, Vermeer SE, van Dijk EJ, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46(12):1604–1610. doi: 10.1007/s00125-003-1235-0. [DOI] [PubMed] [Google Scholar]
- 4.Espeland MA, Miller ME, Go veas JS, et al. Cognitive function and fine motor speed in older women with diabetes mellitus: results from the women's health initiative study of cognitive aging. J Womens Health 2002. 2011;20(10):1435–1443. doi: 10.1089/jwh.2011.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamiyama K, Wada A, Sugihara M, et al. Potential hippocampal region atrophy in diabetes mellitus type 2: a voxel-based morphometry VSRAD study. Jpn J Radiol. 2010;28(4):266–272. doi: 10.1007/s11604-009-0416-2. [DOI] [PubMed] [Google Scholar]
- 6.Novak V, Zhao P, Manor B, et al. Adhesion molecules, altered vasoreactivity, and brain atrophy in type 2 diabetes. Diabetes Care. 2011;34(11):2438–2441. doi: 10.2337/dc11-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14(2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113(6):1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 9.Last D, Alsop DC, Abduljalil AM, et al. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007;30(5):1193–1199. doi: 10.2337/dc06-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manschot SM, Brands AMA, van der Grond J, et al. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55(4):1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- 11.Manschot SM, Biessels GJ, de Valk H, et al. Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia. 2007;50(11):2388–2397. doi: 10.1007/s00125-007-0792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt R, Launer LJ, Nilsson L-G, et al. Magnetic resonance imaging of the brain in diabetes: the Cardiovascular Determinants of Dementia (CASCADE) Study. Diabetes. 2004;53(3):687–692. doi: 10.2337/diabetes.53.3.687. [DOI] [PubMed] [Google Scholar]
- 13.Heikkilä O, Lundbom N, Timonen M, et al. Hyperglycaemia is associated with changes in the regional concentrations of glucose and myo-inositol within the brain. Diabetologia. 2009;52(3):534–540. doi: 10.1007/s00125-008-1242-2. [DOI] [PubMed] [Google Scholar]
- 14.Keymeulen B, Jacobs A, de Metz K, et al. Regional cerebral hypoperfusion in long-term type 1 (insulin-dependent) diabetic patients: relation to hypoglycaemic events. Nucl Med Commun. 1995;16(1):10–16. doi: 10.1097/00006231-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Vázquez LA, Amado JA, García-Unzueta MT, et al. Decreased plasma endothelin-1 levels in asymptomatic type I diabetic patients with regional cerebral hypoperfusion assessed by Spect. J Diabetes Complications. 1999;13(5-6):325–331. doi: 10.1016/s1056-8727(99)00064-1. [DOI] [PubMed] [Google Scholar]
- 16.De Bresser J, Tiehuis AM, van den Berg E, et al. Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care. 2010;33(6):1309–1314. doi: 10.2337/dc09-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu WL, Qiu CX, Wahlin A, et al. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63(7):1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- 18.Mattix HJ, Hsu C, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol JASN. 2002;13(4):1034–1039. doi: 10.1681/ASN.V1341034. [DOI] [PubMed] [Google Scholar]
- 19.Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol JASN. 1996;7(6):930–937. doi: 10.1681/ASN.V76930. [DOI] [PubMed] [Google Scholar]
- 20.Weiner DE, Bartolomei K, Scott T, et al. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis Off J Natl Kidney Found. 2009;53(3):438–447. doi: 10.1053/j.ajkd.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 22.Forman JP, Fisher NDL, Schopick EL, Curhan GC. Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol JASN. 2008;19(10):1983–1988. doi: 10.1681/ASN.2008010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chronic Kidney Disease Prognosis Consortium. Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA J Am Med Assoc. 2001;286(4):421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 25.D'Agostino E, Maes F, Vandermeulen D, Suetens P. Non-rigid Atlas-to-Image Registration by Minimization of Class-Conditional Image Entropy (Internet) In: Barillot C, Haynor DR, Hellier P, editors. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2004. Springer Berlin Heidelberg; 2004. [on 30 January 2014]. pp. 745–753. Accessed at http://link.springer.com/chapter/10.1007/978-3-540-30135-6_91. [Google Scholar]
- 26.Novak V, Last D, Alsop DC, et al. Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care. 2006;29(7):1529–1534. doi: 10.2337/dc06-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Brien LM, Ziegler DA, Deutsch CK, et al. Statistical adjustments for brain size in volumetric neuroimaging studies: some practical implications in methods. Psychiatry Res. 2011;193(2):113–122. doi: 10.1016/j.pscychresns.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knopman DS, Mosley TH, Jr, Bailey KR, et al. Associations of microalbuminuria with brain atrophy and white matter hyperintensities in hypertensive sibships. J Neurol Sci. 2008;271(1-2):53–60. doi: 10.1016/j.jns.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stehouwer CDA, Smulders YM. Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms. J Am Soc Nephrol JASN. 2006;17(8):2106–2111. doi: 10.1681/ASN.2005121288. [DOI] [PubMed] [Google Scholar]
- 31.Joosten H, Izaks GJ, Slaets JPJ, et al. Association of cognitive function with albuminuria and eGFR in the general population. Clin J Am Soc Nephrol CJASN. 2011;6(6):1400–1409. doi: 10.2215/CJN.05530610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartzokis G, Beckson M, Lu PH, et al. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58(5):461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- 33.Franke K, Gaser C, Manor B, Novak V. Advanced BrainAGE in older adults with type 2 diabetes mellitus. Front Aging Neurosci. 2013;5:90. doi: 10.3389/fnagi.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Harten B, de Leeuw FE, Weinstein HC, et al. Brain imaging in patients with diabetes: a systematic review. Diabetes Care. 2006;29(11):2539–2548. doi: 10.2337/dc06-1637. [DOI] [PubMed] [Google Scholar]
- 35.Jassal SK, Kritz-Silverstein D, Barrett-Connor E. A prospective study of albuminuria and cognitive function in older adults: the Rancho Bernardo study. Am J Epidemiol. 2010;171(3):277–286. doi: 10.1093/aje/kwp426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gansevoort RT, Verhave JC, Hillege HL, et al. The validity of screening based on spot morning urine samples to detect subjects with microalbuminuria in the general population. Kidney Int Suppl. 2005;94:S28–S35. doi: 10.1111/j.1523-1755.2005.09408.x. [DOI] [PubMed] [Google Scholar]
- 37.Witte EC, Lambers Heerspink HJ, de Zeeuw D, et al. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol JASN. 2009;20(2):436–443. doi: 10.1681/ASN.2008030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbeau EJ, Ranjeva JP, Didic M, et al. Profile of memory impairment and gray matter loss in amnestic mild cognitive impairment. Neuropsychologia. 2008;46(4):1009–1019. doi: 10.1016/j.neuropsychologia.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 39.Bonekamp D, Yassa MA, Munro CA, et al. Gray matter in amnestic mild cognitive impairment: voxel-based morphometry. Neuroreport. 2010;21(4):259–263. doi: 10.1097/WNR.0b013e328335642a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hämäläinen A, Tervo S, Grau-Olivares M, et al. Voxel-based morphometry to detect brain atrophy in progressive mild cognitive impairment. Neuroimage. 2007;37(4):1122–1131. doi: 10.1016/j.neuroimage.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 41.Kumar A, Haroon E, Darwin C, et al. Gray matter prefrontal changes in type 2 diabetes detected using MRI. J Magn Reson Imaging JMRI. 2008;27(1):14–19. doi: 10.1002/jmri.21224. [DOI] [PubMed] [Google Scholar]
- 42.Zimmerman ME, Brickman AM, Paul RH, et al. The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry. 2006;14(10):823–833. doi: 10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed] [Google Scholar]
- 43.Wong TY, Mosley TH, Jr, Klein R, et al. Retinal microvascular changes and MRI signs of cerebral atrophy in healthy, middle-aged people. Neurology. 2003;61(6):806–811. doi: 10.1212/01.wnl.0000086372.05488.8d. [DOI] [PubMed] [Google Scholar]


