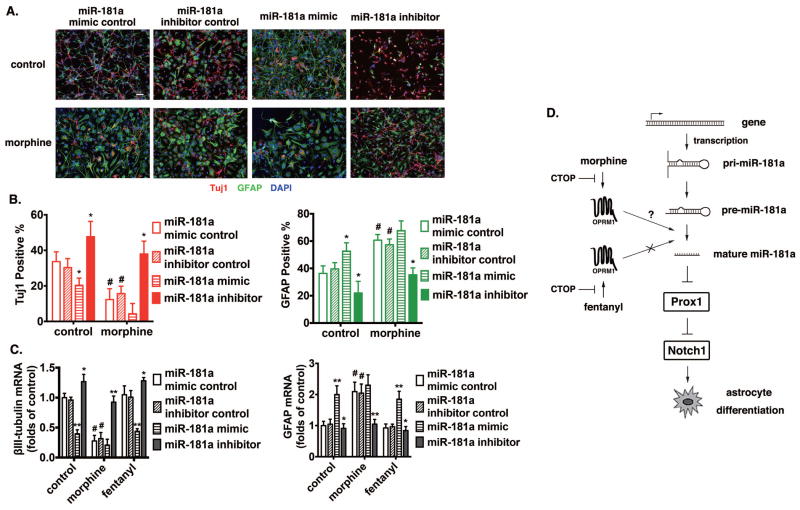
Figure 7. MiR-181a plays an essential role in morphine-induced astrocyte-preferential NPC differentiation.
(A) Mouse NPC primary cultures were transfected with control RNA, miR-181a mimic, or miR-181a inhibitor by using Lipofectamine 2000, with or without the treatment of 1 μM morphine for 4 d. Cells were stained with markers for neurons (Tuj1), astrocytes (GFAP) and with DAPI. Scale bar, 25 μm. Images are representative of at least three independent experiments with similar results.
(B) Quantification of cells stained with each marker, calculated as the percentage of the total number of cells stained with DAPI. Red: Tuj1; Green: GFAP. *, p<0.05 compared to cultures transfected with control RNA with the same treatment. #, p<0.05 compared to the control group transfected with the control RNA. Data are the mean ± SEM of at least three independent experiments.
(C) The expression of βIII-tubulin and GFAP were determined by real-time PCR after 4 d of differentiation. The results were normalized against those of GAPDH, and further normalized against the results obtained from cultures transfected with control RNA in each group. *, p<0.05; **, p<0.01 compared to cultures transfected with control RNA and treated with the same agonist. #, p<0.05 compared to the control group transfected with control RNA. Data represent the mean ± SEM of four independent experiments.
(D) Schematic representation of the miR-181a/Prox1/Notch regulation pathway modulated by OPRM1 activation induced by morphine but not fentanyl.