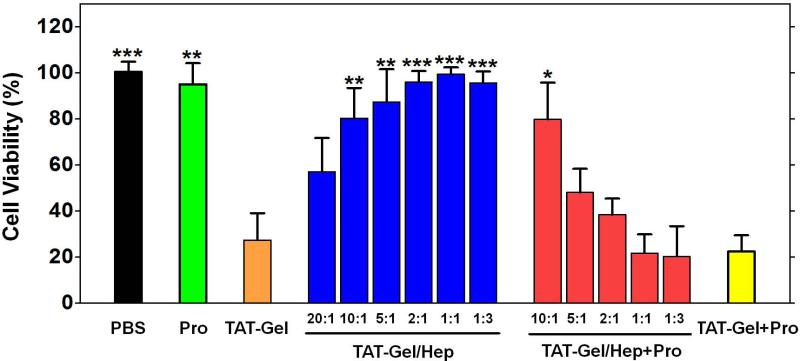
Figure 7.
Heparin/protamine-mediated regulations on cytotoxicity by TAT-Gel. LS174T cells were treated with: 1) PBS, 2) protamine (a.k.a. Pro; 1 μM), 3) TAT-Gel, 4) TATGel/Hep, 5) “TAT-Gel/Hep+Pro” or 6) “TAT-Gel+Pro”, and, after incubation at 37°C for 48 h, the relative cell viability was determined by XTT assay. The concentration of TAT-Gel was 100 nM for all the tested groups. TAT-Gel/Hep complex was prepared by mixing TAT-Gel and heparin in varied molar ratios (20:1, 10:1, 5:1, 2:1, 1:1, 1:3) and incubation at 4°C for 30 min. For “TAT-Gel/Hep+Pro” treatment, protamine was added to TAT-Gel/Hep pre-treated cells (TAT-Gel : heparin = 1:3) with varied heparin : protamine molar ratios (10:1, 5:1, 2:1, 1:1 or 1:3). In case of “TAT-Gel+Pro” treatment, 10-fold molar excess of protamine (final 1 μM concentration) was added to the TAT-Gel pre-treated cells. For all experiments, N=3. Statistical significant differences in the relative cell viability of TAT-Gel-treated group and those of other groups were compared by Student's t-test. * P < 0.01, ** P < 0.001, *** P < 0.0001. (TAT-Gel: recombinant TAT-gelonin fusion protein)