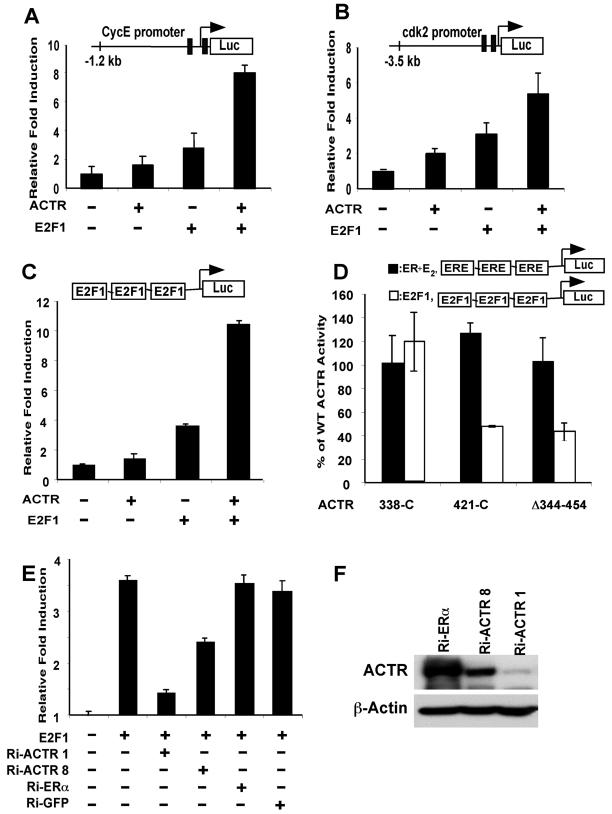
FIG. 7.
ACTR enhances the transactivation by E2F1. (A and B) Activity of ACTR to enhance the E2F1-responsive promoter function was measured in T-47D cells using human cyclin E and cdk2 promoters linked to the luciferase gene. Cells were transiently transfected with the reporters and ACTR or E2F1 expression constructs as indicated. Induction represents the normalized luciferase activity in cells transfected with ACTR and/or E2F1 expression plasmids divided by the activity in cells transfected with the reporter alone. (C) The ability of ACTR to enhance E2F1 transactivation was analyzed using 3x E2F- tk-luc, a reporter with three E2F1 binding sites linked to luciferase gene. 293T cells were transfected with the reporter and plasmids expressing ACTR and/or E2F1. (D) The activity of wild-type and mutant ACTR to enhance E2F1 transactivation was compared with their activity on E2-stimulated ER transactivation. 293T cells were transfected with either 3x E2F-tk-luc or 3x ERE-tk-luc reporter, along with expression plasmids for E2F1 or ERα, and wild-type ACTR or its mutant forms (N-terminal deletions 338-C and 421-C and an internal deletion, Δ344-454). Percentage of wild-type ACTR activity was calculated by dividing the normalized luciferase activity of mutant ACTR with the normalized wild-type ACTR activity. The ERE reporter was analyzed from cells treated with 10−7 M E2. Note that ACTR (wild type) increased E2-stimulated transactivation by ER about fourfold. (E) The requirement for ACTR in E2F1 transactivation was examined in a reporter assay as for panel C, with ACTR expression silenced by vector-mediated RNAi. 293T cells were cotransfected with 3x E2F-tk-luc and an equal amount of vectors expressing sh-RNAi sequences targeting ACTR, ERα, or GFP as indicated. (F) 293T cells were cotransfected with pcD-HCMV-ACTR and the pSh-RNAi vectors as indicated. Cell lysates were prepared 3 days after transfection for Western analysis.