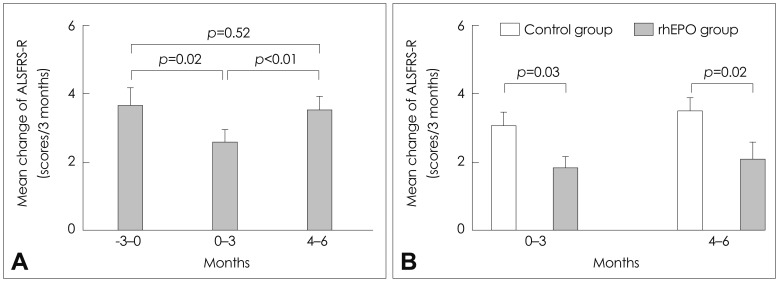
Fig. 2.
Efficacy assessment in studies I and II. A (study I): Comparison of the rates of decline (change in the ALSFRS-R scores over 3 months) during the lead-in (-3-0 months prior to 1 months), treatment (0-3 months), and follow-up (4-6 months) periods. During the treatment period, the rate of decline in the ALSFRS-R score decreased. B (study II): Comparison of the rates of decline in the ALSFRS-R scores in the controls and the rhEPO recipients. The mean rate of decline in the ALSFRS-R score was lower for the rhEPO recipients than for the controls. ALSFRS-R: amyotrophic lateral sclerosis Functional Rating Scale-Revised, rhEPO: recombinant human erythropoietin.