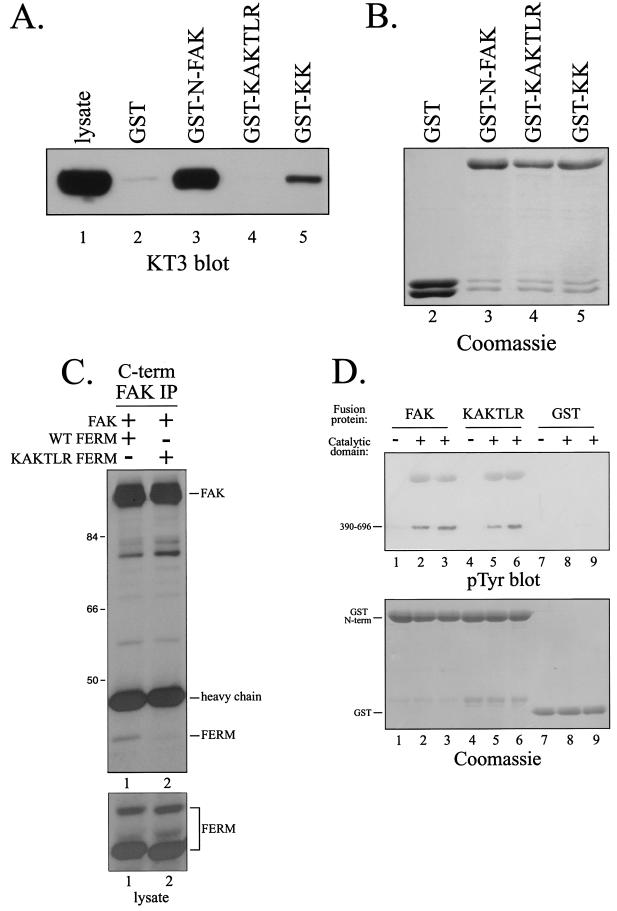
FIG. 4.
Point mutations in the FERM domain disrupt the interaction with FAK. (A) Lysates of CE cells overexpressing epitope-tagged FAK were precleared with GST and then incubated with GST (lane 2), GST-N-FAK (lane 3), GST-KAKTLR (lane 4), or GST-KK (lane 5). Bound FAK was detected by Western blotting with KT3. Lysate (25 μg) was analyzed as a control (lane 1). (B) As a loading control, a fraction of product from each pulldown from panel A was analyzed by SDS-PAGE and Coomassie blue staining. (C) The wild-type (WT) FERM domain (lane 1) or FERM domain with the KAKTLR mutation (lane 2) was transiently coexpressed with full-length, wild-type FAK in HEK 293 cells. FAK was immunoprecipitated with 2A7, and the immune complexes were analyzed by Western blotting with 4.47 (top panel). Lysates were blotted with 4.47 to verify equal expression of the FERM domains (bottom panel). Numbers on the left are molecular weights in thousands. (D) Purified recombinant catalytic domain of FAK was incubated with GST-N-FAK (lanes 2 and 3), GST-KAKTLR (lanes 5 and 6), or GST (lanes 8 and 9) immobilized on glutathione beads in the presence of Mg2+ and ATP. After washing and elution with free glutathione, samples were analyzed by Western blotting for phosphotyrosine (pTyr) (top panel) or by SDS-PAGE and Coomassie blue staining (bottom panel). As controls, GST fusion proteins were also incubated in the absence of the purified recombinant catalytic domain (lanes 1, 4, and 7).