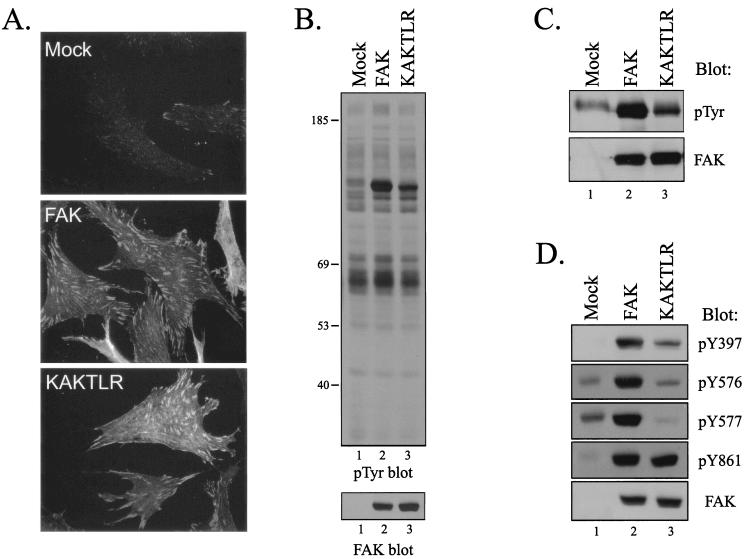
FIG. 5.
KAKTLR is defective for tyrosine phosphorylation in vivo. (A) CE cells (top panel) and CE cells overexpressing wild-type FAK (middle panel) or the KAKTLR mutant (bottom panel) were fixed and stained with the BC4 polyclonal FAK antibody. (B) Upper panel, 25 μg of cell lysate from control transfected CE cells (lane 1) or FAK (lane 2)- or KAKTLR (lane 3)-expressing CE cells was analyzed by Western blotting for phosphotyrosine (pTyr). The positions of molecular weight markers (in thousands) are indicated on the left. Lower panel, the blot was stripped and reprobed for FAK. (C) FAK was immunoprecipitated from lysates of CE cells containing empty vector (lane 1) or expressing FAK (lane 2) or KAKTLR (lane 3). Immune complexes were Western blotted for phosphotyrosine (top panel) and then stripped and reprobed for FAK (bottom panel). (D) Twenty-five micrograms of cell lysate from control transfected CE cells (lane 1) or FAK (lane 2)- or KAKTLR (lane 3)-expressing CE cells was analyzed by Western blotting with phospho-specific antibodies recognizing FAK when phosphorylated on Y397, Y576, Y577, or Y861, as indicated. Lysate was also blotted for FAK to demonstrate equal expression of protein (bottom panel).