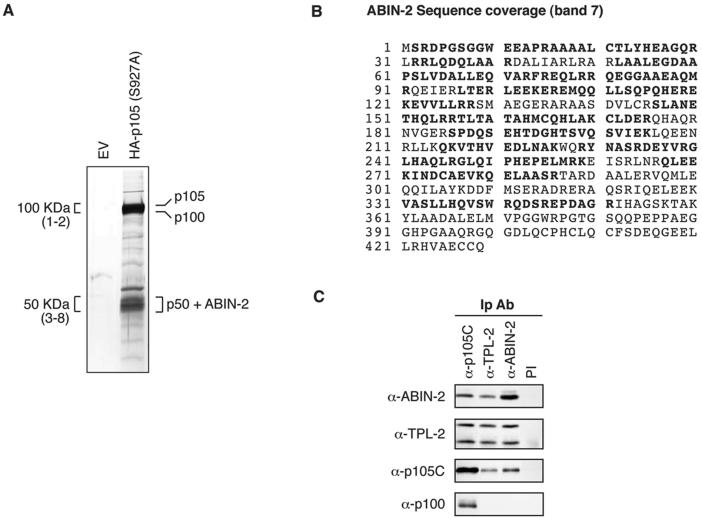
FIG. 1.
ABIN-2 copurifies with NF-κB1 p105. (A) Protein was purified from lysate of HeLa S3 cells stably transfected with HA-p105(S927A) or empty vector (EV) by sequential affinity purification using anti-HA MAb and anti-p105C antibody. Purified protein was resolved by SDS-PAGE (10% acrylamide) and revealed by silver staining. The positions of the identified proteins are shown. For mass spectroscopic analysis, the indicated 100-kDa regions (bands 1 and 2) and 50-kDa regions (bands 3 to 8) were excised as a series of adjacent slices numbered from high to low molecular weight from a replicate SDS-polyacrylamide gel (10% acrylamide) stained with colloidal Coomassie brilliant blue (not shown). Proteins in isolated bands were identified by MALDI mass spectroscopy of tryptic digests. (B) ABIN-2 amino acid sequence showing the deduced portions of peptides (in bold type) identified by MALDI mass spectroscopic analysis of band 7. Peptide coverage corresponded to 56% of the ABIN-2 amino acid sequence. (C) Lysates of HeLa S3 cells were immunoprecipitated with the indicated specific antibodies (IP Ab) or preimmune (PI) IgG. Isolated proteins were resolved by SDS-PAGE (10% acrylamide) and Western blotting. The specificity of blotting and immunoprecipitating antibodies is denoted as α (anti) followed by the name of the recognized antigen.