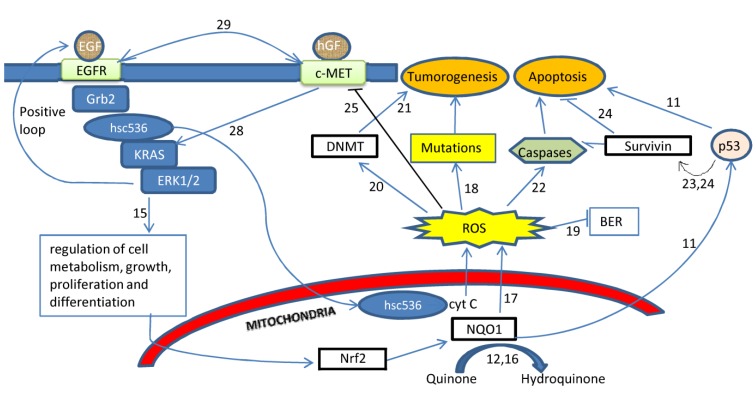
Figure 1.
Inter-relationships among oxidative stress, KRAS, NQO1, survivin, DNA methyltransferases, and c-MET in the pathogenesis of lung cancer a,b.
a See the list of references for literature cited. b Activating mutations in KRAS results in increased cellular growth, metabolism, and proliferation [15]. The reactive oxygen species (ROS) produced during metabolic processes are normally converted into harmless products by antioxidant enzymes such as NQO1 [12,16] which stabilizes the tumor suppressor TP53 [11]. However, when the capacity of NQO1 and other antioxidant enzymes is overwhelmed, the ROS may “leak out” of the mitochondria [17] and oxidize DNA bases, creating mutations [18]. Oxidative stress may repress base excision repair systems, which may result in additional mutations [19]. ROS may increase DNA methyl transferase activity [20], which may be an early event in lung carcinogenesis [21]. ROS regulate apoptosis via upregulation of caspases [22]. Survivin, a caspase inhibitor, also regulated by DNMTs and TP53, may decrease effects of ROS by inhibiting apoptosis [23,24]. Oxidative stress inhibits expression of c-MET, an oncogene altered in many cancers [25,26,27]. c-MET regulates oncogenic transformation of the lung cancers associated with KRAS mutations [28]. Co-activation of c-MET and EGFR is seen in a subset of lung cancers with distinct expression profile, mutational spectrum, and response to chemotherapy [29].