Figure 1.
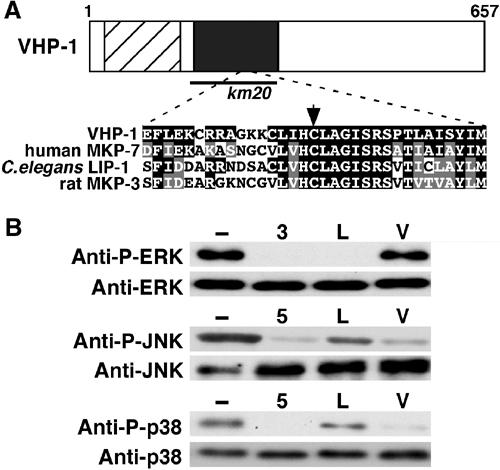
Structure and enzymatic properties of VHP-1. (A) Schematic representation of VHP-1. Hatched and dark boxes represent the rhodanase homology and dual-specificity phosphatase catalytic domains, respectively. The bold line underneath shows the extent of the km20 deletion. Sequence alignment of the catalytic domains of VHP-1, human MKP-7, C. elegans LIP-1, and rat MKP-3 is shown below. Identical and similar residues are highlighted with black and gray shading, respectively. The arrow indicates the essential Cys-262 residue. The DDBJ/EMBL/GenBank number for the VHP-1 sequence is AY585194. (B) In vitro phosphatase assay. Myc-tagged MKPs, rat MKP-3 (3), human MKP-5 (5), LIP-1 (L), and VHP-1 (V), were expressed in COS7 cells and immunoprecipitated with anti-Myc antibody. They were then used for in vitro phosphatase assays utilizing GFP-phospho-ERK, GST-phospho-JNK and GST-phospho-p38 as exogenous substrates. Reaction mixtures were immunoblotted with anti-phospho-MAPKs and anti-MAPKs antibodies. In all experiments, the amounts of immunoprecipitated Myc-MKPs were verified by immunoblotting, confirming that similar amounts of MKPs were used for each assay (data not shown).
