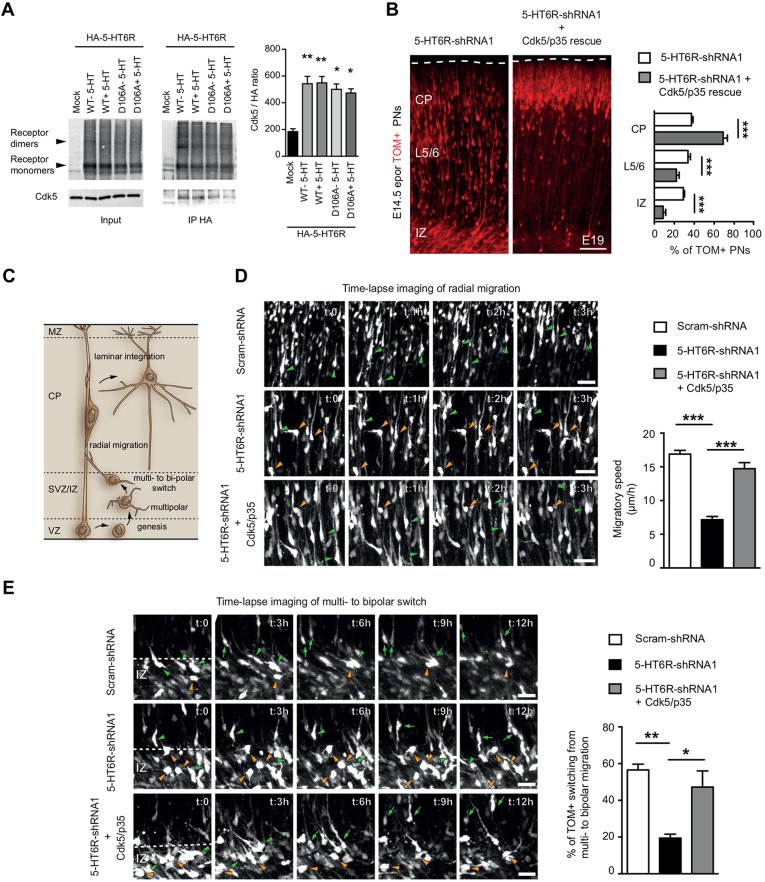
Fig. 3.
5-HT6R controls PN migration through a Cdk5-dependent mechanism. (A) NG108-15 cells were transfected with empty plasmid (mock), with HA-tagged (h)5-HT6R (WT) or a 5-HT6R construct mutated on the serotonin-binding site (D106A) and exposed or not to serotonin (5-HT) (1 µM). The inputs correspond to 5% of the total amount of protein used for immunoprecipitation. Co-immunoprecipitated Cdk5 was detected after HA immunoprecipitation. Quantification of immunoprecipitated Cdk5 indicates a significant increase in the wild-type and D106A conditions compared with the mock control with no effects of serotonin. Data, expressed in arbitrary units, were calculated as ratios of Cdk5 to HA immunoreactive signals in immunoprecipitates. Data are mean±s.e.m. of values obtained in four independent experiments (**P<0.01, *P<0.05, one-way ANOVA, Tukey's post hoc test). (B) In utero electroporation of Cdk5/p35 significantly rescues 5-HT6R-shRNA1-induced mispositioning of PNs compared with scram-shRNA (***P<0.001, unpaired Student's t-test). (C) Two distinct steps in the migration of PNs: the switch from a multi- to bipolar radial migration and radial glial-guided locomotion. (D) Time-lapse imaging showing that locomotion speed of 5-HT6R-shRNA1 TOM+ PNs through deep layers is significantly decreased compared with scram-shRNA and significantly rescued by Cdk5/p35 electroporation. Orange arrowheads indicate cells that remain stationary during the time-lapse sequence, whereas green arrowheads indicate cells that migrate radially. (E) Time-lapse imaging showing that the percentage of 5-HT6R-shRNA1 TOM+ PNs switching from multipolar stage (green arrowheads) to radial migration (green arrows) is significantly decreased versus scram-shRNA and significantly rescued by Cdk5/p35 electroporation. Orange arrowheads indicate multipolar cells that do not switch to radial migration during the time-lapse sequence (***P<0.001, **P<0.01, *P<0.05 one-way ANOVA, Tukey's post hoc test). PNs, pyramidal neurons; MZ, marginal zone; CP, cortical plate; SVZ, subventricular zone; IZ, intermediate zone. Data are mean±s.e.m. Scale bars: 100 µm in B; 40 µm in D; 25 µm in E.