Figure 3.
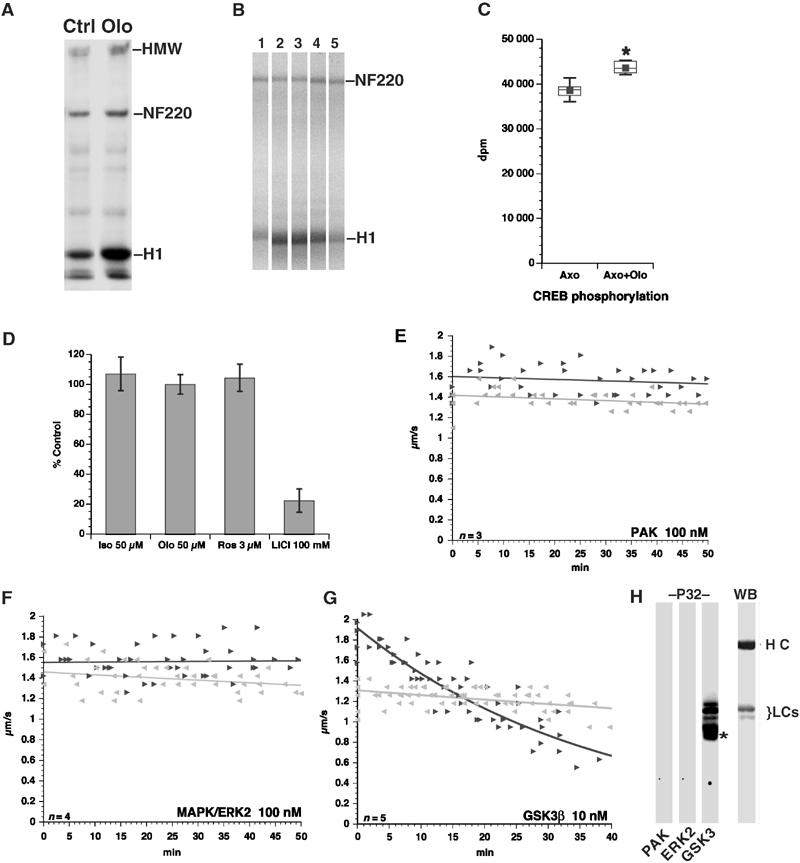
Inhibiting CDK5 activates GSK3. (A) Axoplasms were treated with DMSO (Ctrl) or 5 μM Olo and radiolabeled ATP using histone H1 (H1) as a phosphate acceptor. Autoradiogram shows that Olo increases H1 phosphorylation. Neurofilament heavy chain (NF220) and HMW neurofilament also exhibit increased phosphorylation. (B). Control (1) or Olo-treated (2–5) axoplasms prepared as in (A) were incubated with no peptide (1, 2), ERK peptide (3), CK1 peptide (4) or CREBp (5). Only CREBp prevented Olo-induced increases in histone H1 phosphorylation. (C) GSK3 kinase activity was measured in axoplasm extracts using CREBp as substrate. CREBp phosphorylation increased relative to control axoplasms (Axo) with Olo (Axo+Olo). Increase is significant (P=0.0017; pooled t-test (*)). (D) Effect of 50 μM Iso-Olo (Iso), 50 μM Olo (Olo), 3 μM roscovitine (Ros) and 100 mM LiCl on GSK3 phosphorylation of CREBp in vitro. Values are expressed as percent of GSK3 activity without inhibitors. Vesicle motility assays in isolated axoplasm show effects of PAK (E), ERK2 (F) and GSK3 (G) kinase activities on FAT. Note the specific inhibitory effect on anterograde FAT of GSK3, but not PAK or ERK2, similar to that of CDK5 inhibition. (H) Autoradiogram (P32) shows that GSK3, but not PAK or ERK2, phosphorylates KLCs. Immunoblot (WB) shows position of kinesin heavy (HCs) and light chains (LCs). Asterisk (*) indicates autophosphorylated GSK3.
