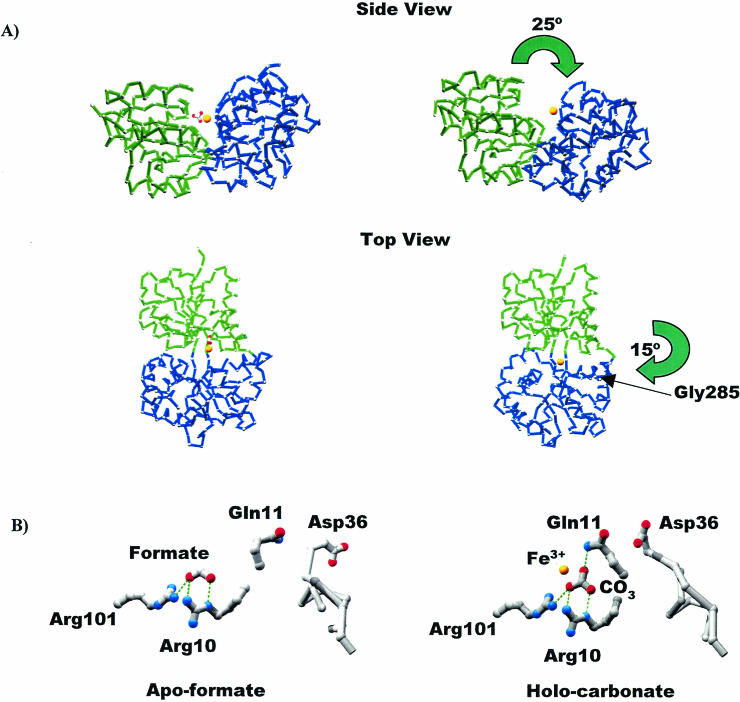
FIG. 4.
Conversion from the closed to the open form of MhFbpA. (A) Cα traces comparing the iron-loaded closed and iron-loaded open forms of the protein. The N-terminal (green) and C-terminal (blue) domains are colored differently to clarify visualization of the domain movements. The domain reorganization can be characterized by two hinge-bending movements: a 25° opening of the N and C lobes through an axis parallel to the ligand-binding cleft, and a 15° rotation through a perpendicular axis through Gly285. The iron atom (yellow sphere) and the carbonate anion are also shown. (B) Comparison of iron-apo-formate and holo-carbonate structures. H bonds are shown as green dotted lines. Formate and carbonate utilize virtually identical binding modes with respect to Arg10, Arg101, and most surrounding elements. Both structures globally adopt closed protein conformations. However, the additional oxygen atom from the carbonate anion faces the solvent-exposed side of the binding pocket, twisting the Gln11 side chain around so that it H bonds with carbonate, which in turn induces a movement in the loop containing Asp36 so that it covers the binding pocket and sequesters the ferric ion from solvent.