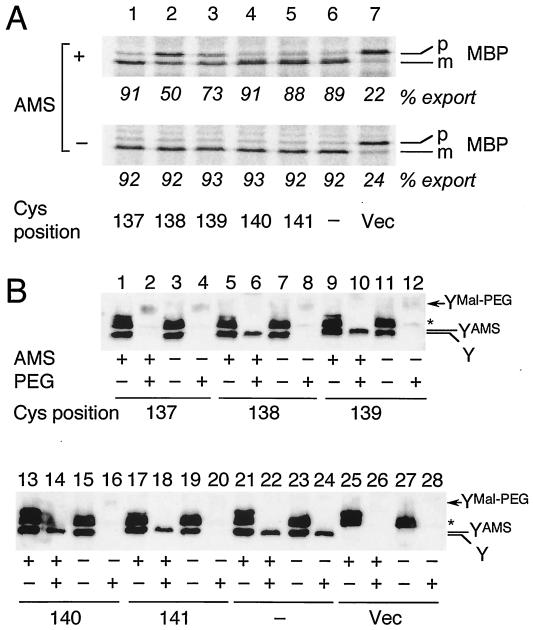
FIG. 4.
(A) Effects of AMS treatment of intact cells on protein export activity. Cells of strain GN31 (secY39) carrying plasmid pSTD343 (lacIq) as well as a plasmid encoding a TM3 single-cysteine SecY derivative (lanes 1 to 5 [Cys substitution positions shown at the bottom of panel A]), cysteine-less SecY-His6-Myc (lane 6), or vector (Vec) (lane 7) were grown at 37°C in M9 minimal medium containing glycerol, amino acids, maltose (0.4%), and IPTG (1 mM) until early log phase. Each culture was divided into two portions, one of which was treated with 3 mM AMS at 37°C for 30 min (+) and not treated with AMS (−). Cells were then pulse-labeled with [35S]methionine for 30 s at 37°C and processed for MBP immunoprecipitation for visualization of labeled MBP species. The positions of the precursor (p) and mature (m) forms of MBP are indicated. The percent export values represent proportions of the mature form. (B) Verification of AMS modification by the blockage of subsequent Mal-PEG modification. Cells expressing SecY with cysteine substitutions, as indicated by the residue numbers, were grown as described above for panel A and treated with AMS as described above (+) or not treated with AMS (−). The samples were precipitated with 5% (final concentration) trichloroacetic acid and solubilized with 1% SDS supplemented with (+) or without (−) 5 mM Mal-PEG, as described in Materials and Methods. After SDS-PAGE, the SecY-His6-Myc proteins were detected by anti-Myc immunoblotting. The positions of SecY-His6-Myc (Y), its AMS-modified form (YAMS), and its Mal-PEG-modified form (YMal-PEG) are indicated. The asterisk marks a nonspecific background protein.