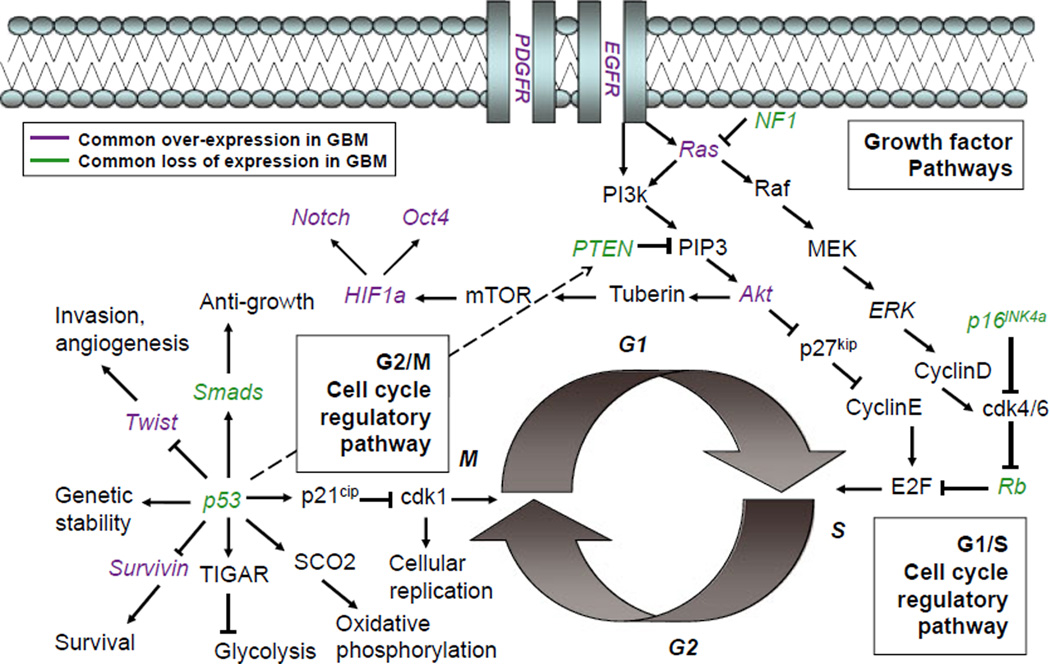
Fig. 1.
Common mutations in glioblastoma are observed throughout cell cycle regulatory pathways. The multifunctional protein p53, which acts as a tumor suppressor regulating the G2/M cell cycle checkpoint, is commonly observed to be lost in patient samples of glioma. MDM2, a regulatory protein that ubiquitinates p53, targeting it for degradation, is observed to be overexpressed in a subset of gliomas; this regulatory protein is itself transcriptionally regulated by p53. Several direct transcriptional and post-translational targets of p53 are commonly affected in glioma. Most other molecular lesions, due to either genetic mutation or expression changes, are observed in the p16/Rb pathway, which regulates the G1/S cell cycle checkpoint. Upon loss of p53, aging neural progenitor cells (NPCs) may be highly dependent on such compensatory pathways to maintain normal cellular activities. This schematic is not an exhaustive description of the complex interactions between many convergent intracellular signaling pathways, but illustrates how a few multifunctional proteins can act as nodes coordinating many cellular activities.