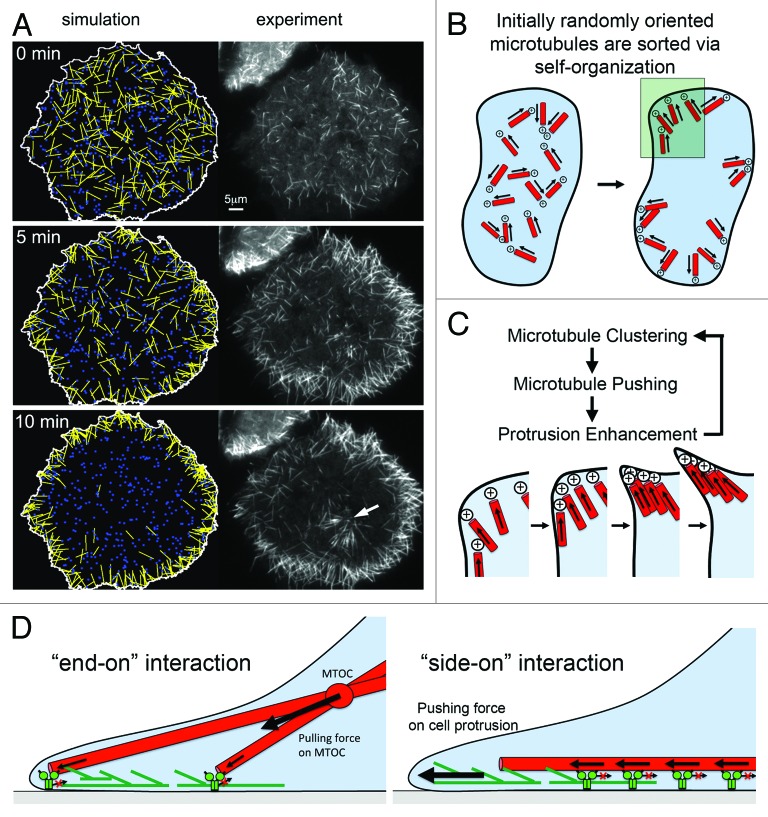
Figure 3. Model for MAP2c and dynein-mediated self-organization of microtubules. (A) Simulations based on mathematical modeling of cortical dynein mediated microtubule transport closely mimic experimental observations after nocodazole washout in the presence of MAP2c. Both the overall redistribution (shown here) and saltatory movements of individual microtubules are observed in those simulations.26 The white arrow points to microtubules radiating from a microtubule organizing center that was not included in simulations. (B) Shortly after washout of nocodaole, small microtubules with random initial orientation nucleate in the cytosol. Those microtubules are transported directionally with leading plus ends via cortical dynein complexes until they encounter an obstacle, which ultimately will be the plasma membrane. This will orient microtubule plus ends to point toward the cell periphery. Microtubules will then push against the outer edge of the cell, which can induce a small convex cell protrusion. (C) Enlarged region marked in B. In a process related to stigmergy, the convex cell protrusion can self-amplify by collecting and trapping more microtubules, which push further against the cell periphery. (D) Distinct modes of interaction between microtubules and cortical dynein. Right: The “end-on” dynein-microtubule interaction produces pulling forces that are integrated to pull the MTOC toward regions of higher cortical dynein density. At homogenous densities, pulling forces can lead to MTOC centering. Left: The “side-on” dynein-microtubule interaction produces pushing forces that are integrated to push microtubules toward the cell periphery where they can stimulate cell protrusions via a “clutch”-like mechanism. Panel A was taken from26 (http://www.molbiolcell.org/content/25/1/95).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.