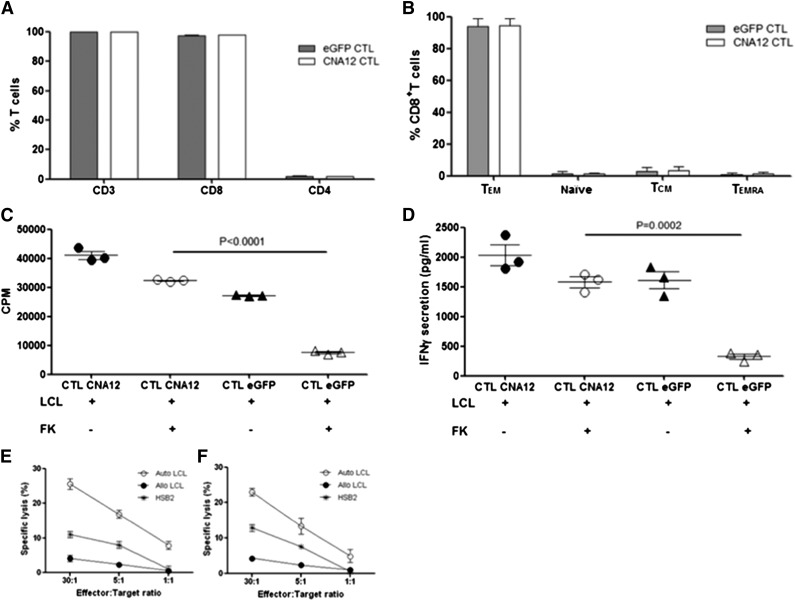
Figure 1.
Immunophenotypic and functional analysis of transduced EBV-specific CTLs before adoptive transfer. (A) CNA12- and eGFP-transduced EBV-CTLs were analyzed for T-cell marker expression by flow cytometry. Percentages of CD3+, CD4+, and CD8+ T cells are shown. (B) Distribution of memory subsets in CNA12- and eGFP-transduced EBV-CTLs. Effector memory (CCR7–CD45RA–), naïve (CCR7+CD45RA+), central memory (CCR7+CD45RA–), terminal-differentiated (TD) effector (CCR7–CD45RA+) T cells. (C) Proliferation ability of EBV-CTLs was evaluated after stimulation in vitro with autologous LCLs with or without therapeutic dose (10 ng/mL) of FK506 and was tested by 3H-thymidine uptake for 21 hours. Proliferation of eGFP-transduced EBV-CTLs after stimulation with LCLs in the presence of FK506 was significantly inhibited (P < .0001) compared with CNA12-transduced EBV-CTLs. (D) EBV-CTLs transduced with CNA12 were able to secrete IFN-γ in the presence of autologous LCLs plus 10 ng/mL of FK506 at comparable levels with those seen with CNA12-transduced T cells stimulated with LCLs alone. eGFP-transduced EBV-CTLs did not produce IFN-γ in the presence of LCLs plus 10 ng/mL FK506 compared with eGFP-transduced T cells stimulated with LCLs alone (P < .001). A standard 51Cr release cytotoxicity assay was performed to assess for the cytotoxicity of EBV-CTL lines against autologous mismatched LCL targets or the T-cell line HSB2 in the presence of 10 ng/mL of FK506. Cytolytic activity of eGFP- (E) and CNA12- (F) transduced cells at different effector-to-target ratios one week after fifth stimulation. The mean ± SEM of 2 CTL lines are shown.