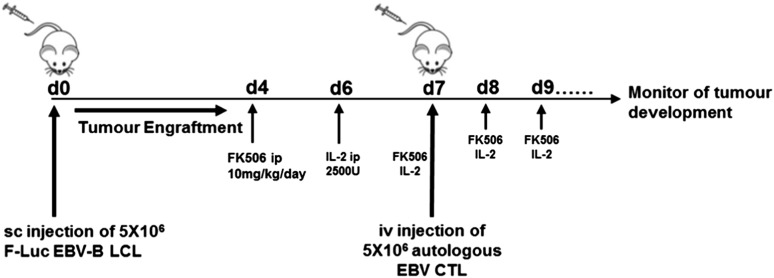
Figure 3.
Experimental design of PTLD xenograft mice model. To perform analysis on the CN-resistant EBV-CTLs in vivo, we investigated their functionality as adoptive therapy in an NSG xenograft model. First, we began generating human EBV-LCLs by infecting PBMCs from EBV-seropositive donor cells with EBV-producing marmoset B-cell line (B95.8) in vitro. Once B-LCL was established, it was used to perform repetitive stimulation of autologous PBMCs to generate EBV-CTLs. For in vivo purposes, we transduced B-LCLs with a retroviral vector encoding FLuc to allow us to monitor tumor development in vivo. Mice were inoculated with 5 × 106 EBV-LCLs subcutaneously on the nape of the neck on day 0 (4 mice/cohort in 2 separate experiments). To assess antitumor activity of control and genetically modified human EBV-CTLs in the presence of FK506 in vivo, mice received 5 × 106 autologous EBV-CTLs IV via the tail vein after 7 days. In addition, mice also received IP injections of 2500 U of IL-2 daily for 7 days post–EBV-CTL. 10 mg/kg body weight of FK506 was also administered IP 5 days per week from 3 to 4 days after LCL injection. Tumor growth was evaluated using the IVIS imaging system. Photon emission from FLuc+ LCLs was quantified using Living Image software.