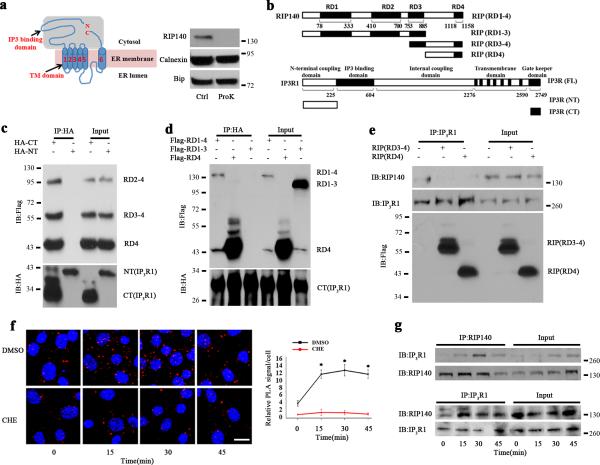
Figure 3. RIP140 interacts with IP3R1.
a, Western blot analysis of RIP140 in ER fraction treated with proteinase K (ProK) or without the enzyme (Ctrl). b, Constructs of RIP140 and IP3R1. c, Reciprocal coimmunoprecipitation examining the interaction domain of IP3R1 with RIP140. HT22 cells were transfected with HA-tagged N-terminal (NT) or C-terminal (CT) domain of IP3R1 and flag-tagged RD2-4, RD3-4 and RD4 of RIP140. By coimmunopreciping with HA antibody, the flag-tagged domains of RIP140 can be detected only in the presence of CT. d, Reciprocal coimmunoprecipitation examining the interaction domain of RIP140 with IP3R1. HT22 cells were transfected with HA-tagged C-terminal (CT) of IP3R1 and flag-tagged RD1-4, RD4 and RD1-3 of RIP140. By coimmunopreciping with HA antibody, only the RD4-containing flag-tagged RIP140 can be detected. e, Competition of RD4, or RD4-containing RD(3-4), with the endogenous RIP140 for interacting with IP3R1 in HT22. Cells were transfected with control vector, RD3-4 and RD4, the endogenous IP3R1-interacting RIP140 was detected by RIP140 antibody that can only recognize N-terminal domain of RIP140. RD4 domain of RIP140 competes the binding of RIP140 to IP3R1. f, Increased association of endogenous RIP140 with IP3R1 monitored by in situ PLA assay in HT22 cells treated with thapsigargin for different durations. The red puncta show endogenous RIP140-IP3R1 complexes. The graph shows statistical results presented as means ± SEM. from five independent experiments; *p<0.05 relative to the control group as determined by Student's t-test. Scale bar, 20 μm. g, Coimmunoprecipitation examining binding of RIP140 to IP3R1 in HT22 following Tg treatment.