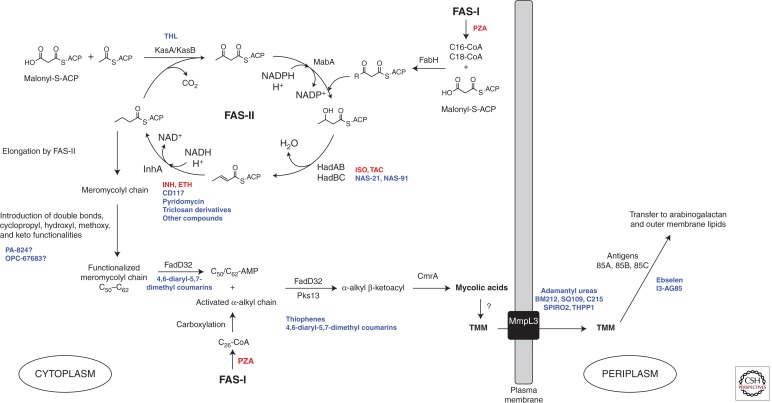
Figure 4.
Biosynthetic pathway of mycolic acids in Mtb and site of action of anti-TB drugs. The saturated α-alkyl chain (C24 or C26 in Mtb) and the meromycoloyl chain (C50–C62) are synthesized by fatty-acid synthase-I (FAS-I) and fatty-acid synthase-II (FAS-II), respectively. The initial substrates of FAS-II are medium length keto-acyl-ACP resulting from the condensation by the Mtb FabH protein of the acyl-CoA products of FAS-I with malonyl-ACP. After reduction by the β-keto-acyl-ACP reductase MabA, dehydration by the (3R)-hydroxyl-dehydratases HadAB and HadBC and reduction by the enoyl-CoA reductase InhA, either the β-ketoacyl-ACP synthase KasA or KasB catalyzes the condensation of the resulting product with malonyl-ACP units, thereby initiating the next round of elongation. Methyltransferases and other as yet unknown enzymes modify the meromycolyl chain introducing double bonds, cyclopropyl, hydroxy, methoxy, and keto functionalities. These two chains are coupled together via Claisen condensation by the acyl-AMP ligase FadD32 and the polyketide synthase Pks13. Upon release from Pks13, reduction by CmrA yields mycolic acids, which are then translocated to the periplasm under the form of TMM by partially elucidated translocation machinery involving the essential integral membrane transporter, MmpL3, and attached to AG or another molecule of TMM to form TDM, by the antigen 85 complex. The sites of action of Mtb inhibitors are indicated. Drugs presently or formerly used in the clinical treatment of TB are in red; inhibitors under development are in blue (see Table 1). THL, thiolactomycin; PZA, pyrazinamide.