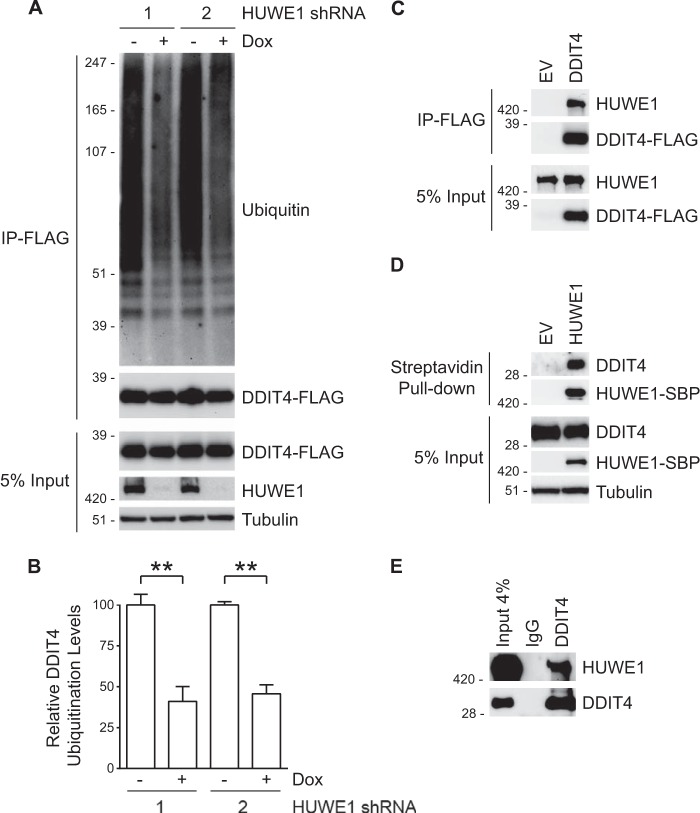
FIGURE 3.
DDIT4 interacts with HUWE1, and its ubiquitination status is regulated by the E3. A, HeLa cells stably expressing two unique Dox-inducible HUWE1 shRNAs were treated in the absence and presence of Dox for 48 h, transfected with FLAG-tagged DDIT4 for 24 h, and incubated with MG132 for an additional 6 h. Input whole cell lysates were subjected to immunoprecipitation (IP) with FLAG resin, and samples were analyzed by immunoblotting. B, quantification of ubiquitinated DDIT4-FLAG from three assays as described in A. Data are represented as the means ± S.E. with p values determined using Student's unpaired t test (**, p < 0.005). Error bars represent S.E. C, HeLa cells were transfected with DDIT4-FLAG or an empty vector (EV) control for 24 h. Following immunoprecipitation with FLAG resin, samples were analyzed by immunoblotting. D, immunoblot analysis of lysates from HeLa cells transfected with SBP-tagged HUWE1 or empty vector (EV) for 24 h and subjected to streptavidin pull-down. E, endogenous HUWE1 can be immunoprecipitated with anti-DDIT4 resin from HeLa cell extracts. Rabbit IgG served as a control.