FIGURE 4.
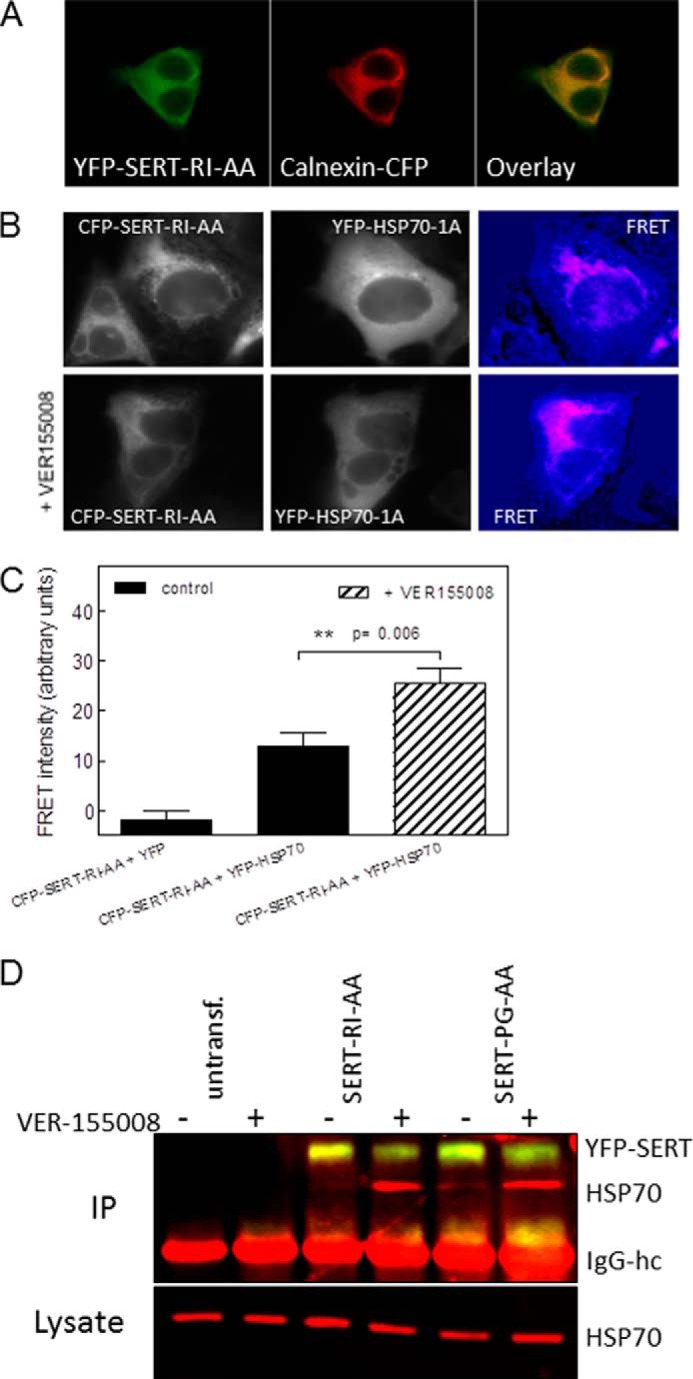
Trapping of HSP70-1A by the inhibitor VER-155008 enhances the association with ER-resident folding-deficient mutant SERTs. A, CFP-tagged calnexin (Calnexin-CFP) was coexpressed with YFP-tagged SERT-R607A/I608A (YFP-SERT-RI-AA) in HEK293 cells. CFP and YFP images were captured with a Zeiss LSM 510 confocal microscope (argon laser, 30 milliwatts; helium/neon laser, 1 milliwatt) equipped with an oil immersion objective (Zeiss Plan-Neofluar ×40/1.3) at 458 and 514 nm with 6% laser power and a pinhole size of 2.5 μm. The Zeiss LSM Image Browser (Version 4.2.0.121, Carl Zeiss Microimaging) was used to analyze the images. The merged image (right panel) was generated to visualize co-localization of the proteins. B, FRET between ER-retained CFP-SERT-R607A/I608A (CFP-SERT-RI-AA) and YFP-HSP70-1A in the absence (top row) and presence of VER-155008 (bottom row). HEK293 cells were transfected, and FRET was recorded as described in the legend to Fig. 2; cells were preincubated with VER-155008 (40 μm) for 2 h prior to capturing the images. C, FRET intensities recorded in cells co-transfected with CFP-SERT-R607A/I608A and YFP or YFP-HFSP1A in the absence and presence of VER-155008 were quantified in three independent transfections with 16, 16, and 20 images captured, respectively. The statistical comparison between FRET intensities recorded in the absence and presence of VER-155008 was done using an unpaired Student's t test. Error bars represent S.E. D, untransfected (untransf.) HEK293 cells and HEK293 cells expressing YFP-tagged SERT-R607A/I608A (SERT-RI-AA) or SERT-P601A/G602A (SERT-PG-AA) were incubated in the absence (lanes labeled “−”) and presence of 40 μm VER-155008 (lanes labeled “+”) for 2 h followed by cross-linking. Cellular lysates were prepared and incubated with an antibody directed against GFP to immunoprecipitate SERT. In the upper blot, immunoprecipitated (IP) SERT and HSP70-1A were simultaneously visualized with antibodies against GFP and HSP70-1A and fluorescent secondary antibodies against murine (red) and rabbit IgG (yellow). The position of the IgG heavy chain (IgG-hc) is also indicated. The cellular levels of HSP70-1A were determined by blotting an aliquot (0.5%) of the lysate (lower blot). The experiment was reproduced twice with similar results.
