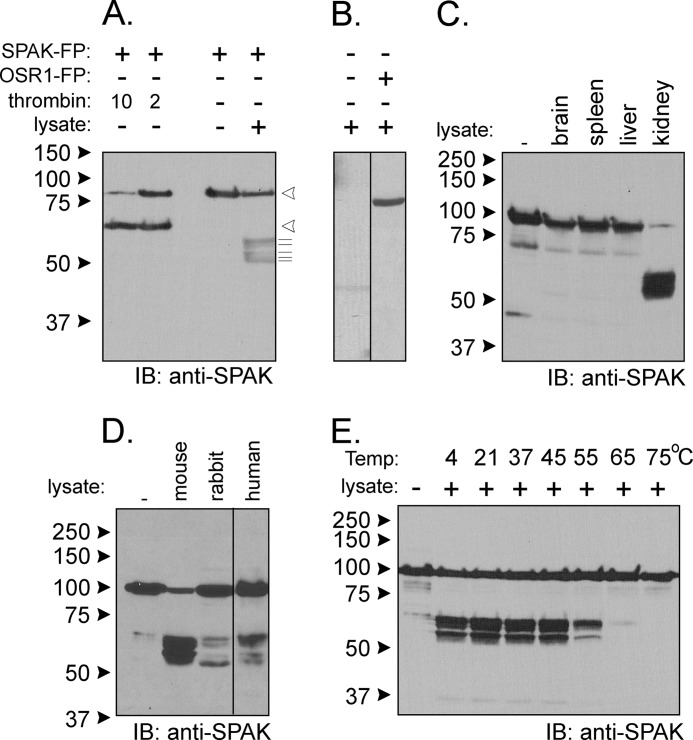
FIGURE 2.
Kidney lysate cleaves SPAK fusion protein. A, the addition of kidney lysate (70 μg) to SPAK fusion protein (SPAK-FP, 10 μg) and subsequent reaction for 1 h at 37 °C resulted in the appearance of several bands above the 50-kDa mark. The pattern is different from thrombin proteolytic cleavage. Open arrowheads indicate sizes of the full-length fusion protein and C-terminal fragment after thrombin. The thin lines under arrowheads highlight the presence of at least five fragments of B. IB, immunoblot. The pattern was not observed without SPAK fusion protein and was also not observed for reaction between kidney lysate and GST-OSR1 (10 μg) fusion protein. C, reaction between SPAK fusion protein and different tissue lysates. All reactions were performed with identical protein amounts of tissue lysate (100 μg). IB, immunoblot. D, proteolytic cleavage from mouse, rabbit, and human kidney lysates (70 μg). E, effect of temperature on the reaction. Two different protocols were used. For temperatures of 4, 20, and 37 °C, kidney lysates were added to GST-SPAK fusion protein, and a subsequent reaction was carried out at these temperatures. For temperatures higher than 37 °C, kidney lysate was first preincubated at these temperatures before reacting at 37 °C with GST-SPAK fusion protein. Western blot analyses were done using 1:1000 anti-SPAK (C-terminal) antibody followed by 1:5000 HRP-conjugated anti-rabbit antibody.