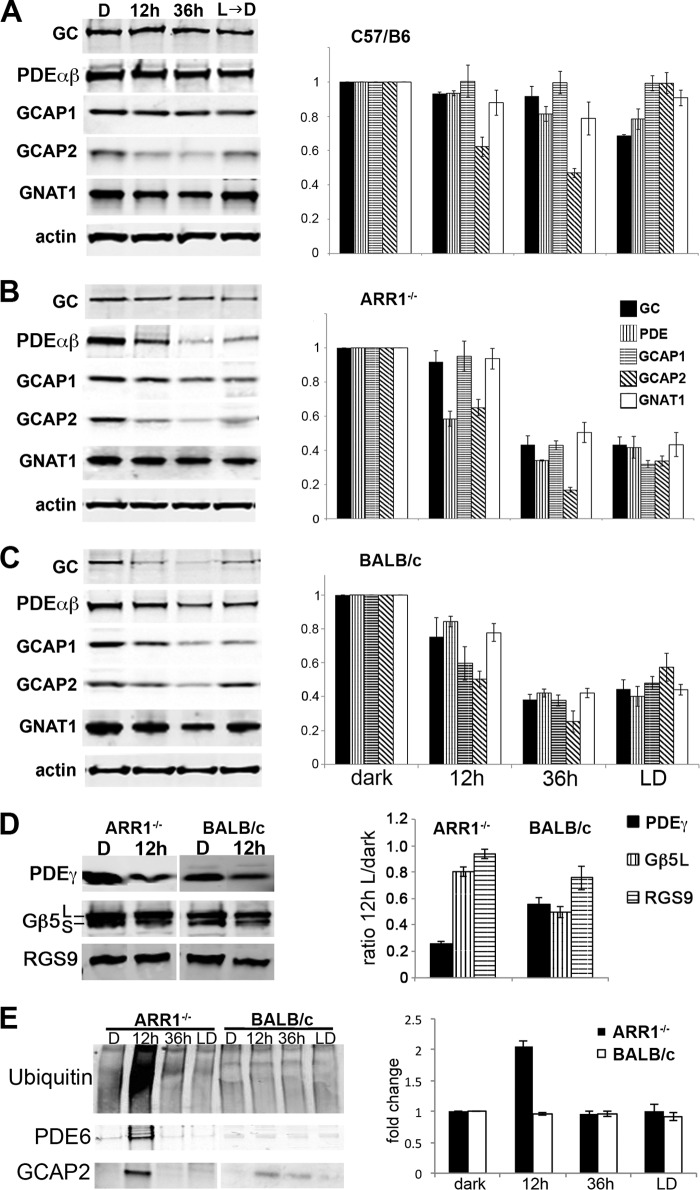
FIGURE 2.
A–C, distinct degradation profiles of phototransduction proteins in light-exposed C57/B6 (A), ARR1−/− (B), and BALB/c (C) mice. Light exposure conditions were the same as in Fig. 1. The left panels show Western blots of GC1, PDE6αβ, GCAP1, GCAP2, and the α-subunit of rod transducin (GNAT1) in retinal extracts obtained from the indicated mice. Relative fold changes of these proteins normalized to the dark-adapted values, as well as levels of actin is shown in the bar graphs (mean ± S.E., n ≥ 3). D, Western blots of the GTPase-activating protein proteins PDE6γ, Gβ5, and RGS9 (left panel). Both the Gβ5L and the Gβ5S isoforms were recognized by the Gβ5 antibody. The signals were quantified and normalized to the dark-adapted values and actin levels as described above (mean ± S.E., n ≥ 3). E, global increase in ubiquitinated proteins was observed after 12 h of light exposure in ARR1−/− retinas but not in BALB/c retinas. A proteasome pull down of retinal extracts obtained after 12 h of light exposure showed PDE6αβ and GCAP2 to be associated with this protein degradation machinery in the ARR1−/− sample. The BALB/c extract also showed low levels of GCAP2 associated with the proteasome. The bar graph shows the relative fold change of protein ubiquitination levels compared with unexposed control levels in light-exposed ARR1−/− and Blab/C retinas (means ± S.E., n ≥ 3).