FIGURE 5.
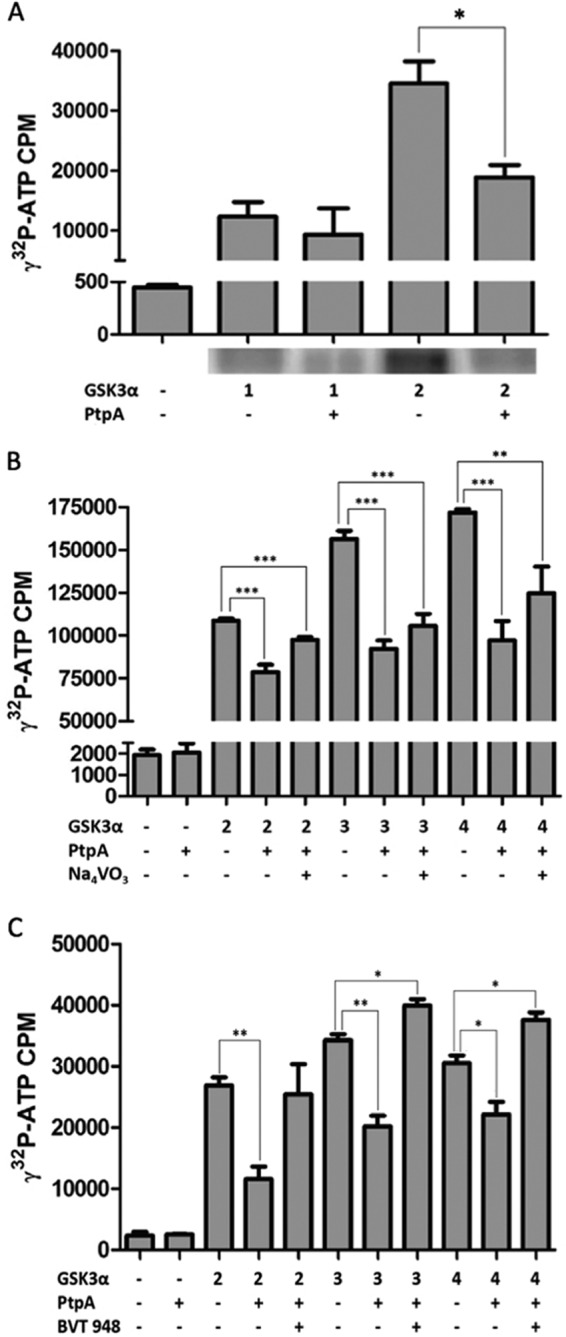
Kinase assays of PtpA dephosphorylation of GSK3α in vitro. A, dephosphorylation of GSK3α by PtpA was tested in an in vitro kinase assay. GSK3α (1 and 2 μm) was autophosphorylated in a kinase buffer containing 10 μCi of [γ-32P]ATP with or without PtpA (0.04 μm) and was resolved onto a 12% SDS gel and exposed to a PhosphorImager screen for radiolabeled band localization. After drying the gel, bands corresponding to phosphorylated GSK3α were cut from the gel, and the radioactive incorporation was measured by a scintillation counter. This graph represents the radioactivity of the dried gel. Results are expressed as ± S.D. of three independent experiments. The p value of 2 μm GSK3α with PtpA is 0.0214. B and C, inhibiting effect of PtpA on GSK3α's activity was tested by radiometric analysis. GSK3α (2–4 μm) was autophosphorylated in a reaction containing kinase buffer and 10 μCi of [γ-32P]ATP. PtpA (0.04 μm) and Na3VO4 (1.5 mm) or PtpA (0.04 μm) and BVT 948 (5 μm) were added to the sample mixtures and were spotted onto phosphocellulose paper. Radioactivity levels were measured by a scintillation apparatus. *, p < 0.05; **, p < 0.001; ***, p < 0.0001. Significant difference is compared by Student's t test.
