FIGURE 3.
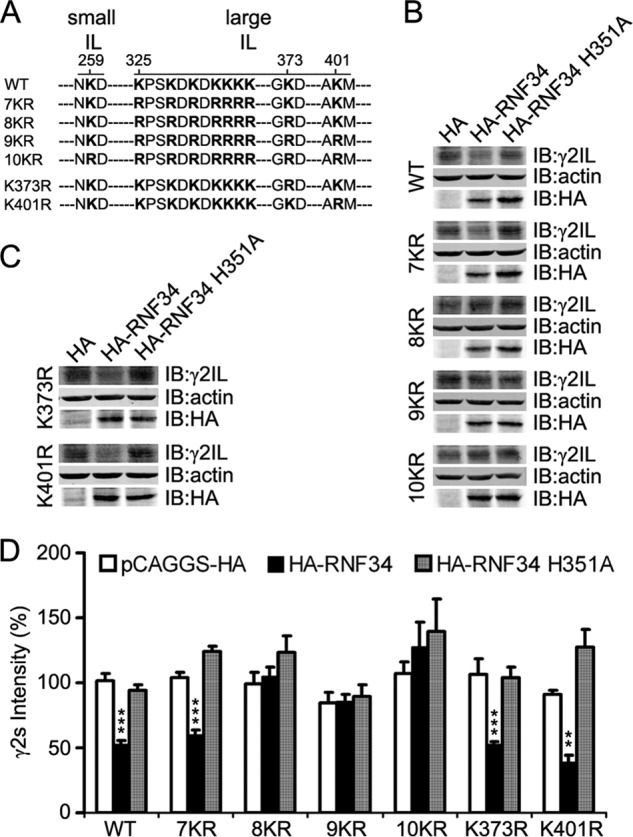
Combination of several lysine-to-arginine mutations in the IL makes the γ2s subunit resistant to the degradation by RNF34. A, illustration of the position of the lysine residue at the small intracellular loop (small IL, aa 259) and of the 9 lysine residues at the large intracellular loop (large IL, aa 325, 328, 330, 332, 333, 334, 335, 373, and 401) of the γ2s subunit that were mutated into arginines. The small IL (aa 258–260) is located between TM1 and TM2, and the large IL (aa 313–404) is between TM3 and TM4. B, HA-RNF34 significantly reduces the expression level of γ2s WT or 7KR, but it does not significantly affect the expression level of γ2s 8KR, 9KR, or 10KR, compared with the HA or HA-RNF34 H351A controls. HEK293 cells, co-transfected with a γ2s subunit (WT, 7KR, 8KR, 9KR, or 10KR), ubiquitin, and a RNF34 construct (HA-RNF34 or HA-RNF34 H351A) or pCAGGS-HA as control vector were lysed, subjected to SDS-PAGE, and immunoblotted (IB) with Ms anti-γ2IL, Ms anti-actin, or Ms anti-HA. C, expression level of γ2s K373R or K401R point mutants (the Lys-Arg point mutation of the 8th or 9th lysine residues in the large IL of γ2s subunit) was significantly reduced by HA-RNF34, but not by HA-RNF34 H351A. D, quantification of the γ2s subunit protein band intensity normalized for that of actin and pCAGGS-HA transfection control for WT. Data are presented as mean ± S.E., n = 4 independent experiments. **, p < 0.01; ***, p < 0.001, for each mutant, statistical significance was determined in one-way ANOVA Tukey-Kramer multiple comparison test.
