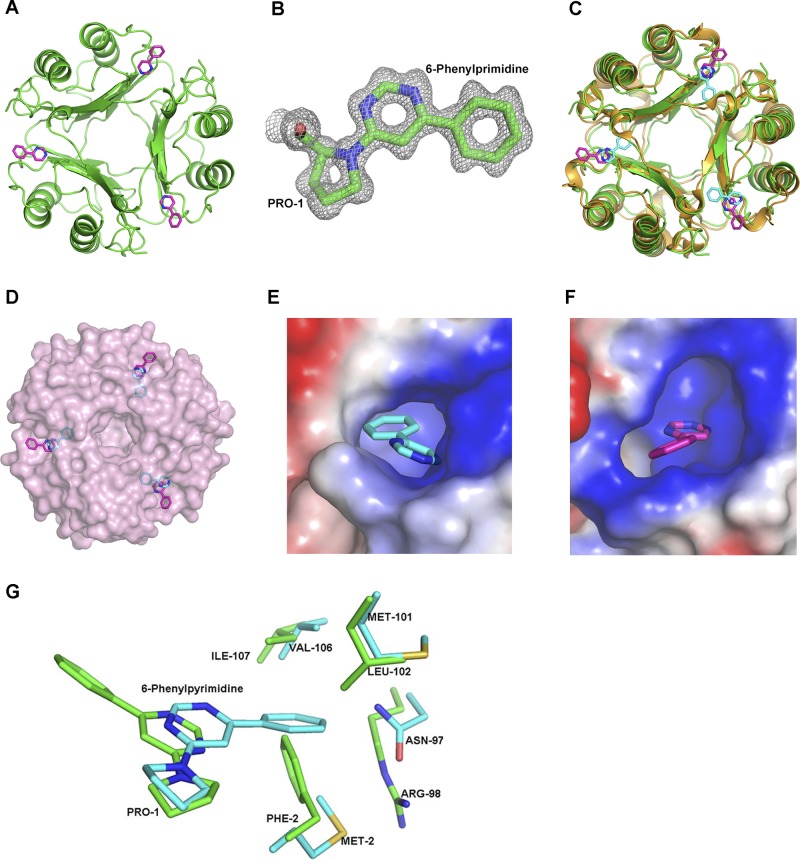
Figure 3.
Structure of D-DT–6-PP, comparison to MIF–6-PP, and structural models of ISO-1 and HPP in complex with D-DT. A) Representation of the 3-dimensional structure of trimeric hD-DT (green) in a covalent complex with 6-PP (pink). (See also Supplemental Fig. S3.) B) Electron density map (2Fo − Fc) of 6-PP covalently bonded to Pro-1 in D-DT contoured at 1σ. C) Superposition based on Cα atoms of human MIF–6-PP (gold–cyan) and hD-DT–6-PP (green–pink). The overall topology of the human MIF–6-PP complex superposed well with the hD-DT–6-PP complex. D) Different orientation of the 6-PP covalent adduct in D-DT (pink) and MIF (cyan) after superposition of the 2 proteins viewed down the 3-fold axis in the context of the solvent-exposed surface area. 6-PP of D-DT is outside the pocket, whereas the 6-PP of MIF is buried inside the active site. E, F) Electrostatic potential map of the D-DT (E) and MIF (F) active site complexed to 6-PP. The active site of D-DT has a greater positive potential compared to MIF (see Supplemental Fig. S4). G) Comparison of the active site atoms of D-DT (green) and MIF (cyan) with the 6-PP covalently bound form. MIF–6-PP aligned onto D-DT–6-PP showed different conformations of 6-PP buried inside the active site of MIF and at the surface of the pocket for D-DT. 6-PP was precluded from binding to the active site D-DT (green) structure due to close contacts by Phe-2 and Arg-98.