Abstract
Intense swimming causes circulatory lactate accumulation in rainbow trout because lactate disposal (Rd) is not stimulated as strongly as lactate appearance (Ra). This mismatch suggests that maximal Rd is limited by tissue capacity to metabolize lactate. This study uses exogenous lactate to investigate what constrains maximal Rd and minimal Ra. Our goals were to determine how exogenous lactate affects: 1) Ra and Rd of lactate under baseline conditions or during graded swimming, and 2) exercise performance (critical swimming speed, Ucrit) and energetics (cost of transport, COT). Results show that exogenous lactate allows swimming trout to boost maximal Rd lactate by 40% and reach impressive rates of 56 μmol·kg−1·min−1. This shows that the metabolic capacity of tissues for lactate disposal is not responsible for setting the highest Rd normally observed after intense swimming. Baseline endogenous Ra (resting in normoxic water) is not significantly reduced by exogenous lactate supply. Therefore, trout have an obligatory need to produce lactate, either as a fuel for oxidative tissues and/or from organs relying on glycolysis. Exogenous lactate does not affect Ucrit or COT, probably because it acts as a substitute for glucose and lipids rather than extra fuel. We conclude that the observed 40% increase in Rd lactate is made possible by accelerating lactate entry into oxidative tissues via monocarboxylate transporters (MCTs). This observation together with the weak expression of MCTs and the phenomenon of white muscle lactate retention show that lactate metabolism of rainbow trout is significantly constrained by transmembrane transport.
Keywords: lactate fluxes, glycolysis, carbohydrate metabolism and fish exercise, tracer methodology and respirometry, Oncorhynchus mykiss
lactate is a particularly dynamic intermediate of carbohydrate metabolism because it plays many roles as an oxidative fuel, glycolytic end-product, gluconeogenic precursor, and intracellular signaling molecule (6, 12, 29). Rainbow trout supports high baseline lactate fluxes of ∼20 μmol·kg−1·min−1 and double lactate production during hypoxia or intense exercise (28, 34). Both of these stresses cause the accumulation of lactate in the blood because the rate of disposal from the circulation (Rd lactate) is not stimulated as much as the rate of appearance in the circulation (Ra lactate). This mismatch suggests that maximal Rd is limited by the capacity of tissues to metabolize lactate through oxidation (red muscle, heart, gill, and brain) and gluconeogenesis (liver). If this hypothesis is correct, the administration of exogenous lactate should not lead to an increase in Rd above the maximal values of 30–35 μmol·kg−1·min−1 already observed after hypoxia or swimming (28, 34). Some fish species, like rainbow trout and plaice, retain lactate in white muscle after exhausting exercise (36, 38, 39). Recent characterization of monocarboxylate transporters (MCTs) in rainbow trout has revealed a low expression of their mRNA in white muscle (particularly the main lactate exporter: MCT4), and a total absence of upregulation even after intense exercise (27). It is unclear how an animal expressing very low levels of MCTs and exhibiting white muscle lactate retention would be able to deal with exogenous lactate.
Previous studies investigating the effects of exogenous lactate administration have been only carried out on humans. Extra lactate provided intravenously can cause a 10% increase in resting metabolic rate, but it does not help or impair athletic performance (9, 11, 13). However, a study comparing different sports drinks showed that lactate ingestion can increase capacity for high-intensity work (1). In exercise experiments in which circulating lactate levels were maintained elevated (lactate clamp), it was demonstrated that lactate oxidation was increased, whereas glucose oxidation was decreased (23, 24). No information is available for fish, but exogenous lactate may impact swimming capacity in two ways. It could improve the ability for intense exercise by providing more carbohydrate to working muscles to oxidize, in addition to endogenous glucose and glycogen. Alternatively, accumulating what some consider a dead-end waste product of glycolysis could decrease work capacity through acid-base disturbance. Therefore, supplying exogenous lactate to an exercising fish could affect its critical swimming speed (Ucrit) and its cost of transport (COT), particularly if extra energy was required to clear the end product.
Previous studies investigating the effects of exogenous supply on lactate kinetics report conflicting results, and it is unclear whether endogenous lactate production can respond to changes in circulating lactate availability. Searle et al. (30) gave 20 or 30 μmol·kg−1·min−1 extra lactate to resting human subjects and observed a 64% decrease and a total inhibition of Ra, respectively (30). By contrast, Jenssen et al. (19) measured no significant change in resting Ra when infusing lactate at a higher rate of 40 μmol·kg−1·min−1, and Miller et al. (23) showed that a lactate clamp causes no change in the Ra lactate of resting or exercising subjects. Therefore, the aim of the present study was to quantify the effects of exogenous lactate on the lactate kinetics and the swimming capacity of rainbow trout. More specifically, our goals were 1) to quantify how exogenous lactate influences the rates of endogenous lactate disposal (Rd) and appearance (Ra) under baseline conditions or during graded swimming, and 2) to determine whether providing extra lactate would affect exercise performance (Ucrit) and locomotion energetics (COT). We anticipated that the higher availability of this preferred oxidative fuel (4, 20, 25, 32) would increase Ucrit and inhibit baseline Ra lactate, but that it would not stimulate Rd lactate beyond the maximal values previously observed after hypoxia and intense exercise.
METHODS
Animals.
Rainbow trout (Oncorhynchus mykiss Walbaum) of both sexes (380 ± 14 g; n = 29) were purchased from Linwood Acres Trout Farm (Campbellcroft, ON, Canada) and held in a 1,300-liter flow-through tank in dechlorinated, well-oxygenated water at 13°C under a 12:12-h light-dark photoperiod. The animals were acclimated to these conditions for at least 2 wk before experiments. They were fed floating fish pellets (Martin Mills, Elmira, Ontario, Canada) three times a week to satiation. They were randomly divided into two groups to measure the effects of exogenous lactate administration 1) on swimming performance (Ucrit, total and net costs of transport: TCOT and NCOT) (n = 17), and 2) on lactate kinetics at rest and during graded exercise (n = 12). All procedures were approved by the Animal Care Committee of the University of Ottawa and adhered to the guidelines established by the Canadian Council on Animal Care.
Catheterizations.
Before surgery, the fish were fasted for 24 h and anesthetized with ethyl 3-aminobenzoate methanesulfonate (MS-222; 60 mg/l) in well-oxygenated water. They were cannulated in the dorsal aorta (BPE-T50 catheters; Instech Laboratories, Plymouth Meeting, PA) following the procedure of Haman and Weber (16). During surgery, the anesthetic solution was recirculated and aerated to perfuse the gills. The first catheter was inserted into the artery at the third gill arch, and the second catheter was inserted at the first gill arch. For lactate kinetics experiments, one catheter was used to infuse lactate (labeled tracer and exogenous unlabeled lactate), while the other was used for blood sampling. Animals used to measure swimming performance were fitted with a single catheter inserted into the artery at the first gill arch for the administration of saline (controls) or exogenous lactate. Only animals with a hematocrit of >20% after recovery from surgery were used in experiments. The catheters were kept patent by flushing with Cortland saline (41) containing 50 U/ml heparin. They were made accessible through water-tight ports in the respirometer lids.
Respirometry, exogenous lactate infusions, and Ucrit protocol.
After surgery, each animal was allowed to recover overnight in a 13.6-liter, cylindrical respirometer (resting experiments), or in a 90-liter swim tunnel respirometer (exercise experiments) (Loligo Systems, Tjele, Denmark). Respirometers were supplied with the same quality water as the holding tank. Metabolic rate (MO2) was measured by intermittent flow respirometry as previously described (34). The effects of exogenous lactate were assessed by comparing control animals receiving saline infusions with test animals receiving lactate infusions from a calibrated syringe pump (Harvard Apparatus, South Natick, MA; 1 ml/h). Exogenous Na-lactate was infused at a rate of 30 μmol·kg−1·min−1 or twice the baseline rate of endogenous lactate production measured in a previous study (28). Control fish were infused with Cortland saline containing matching amounts of sodium. For all swimming experiments, the fish performed a stepwise Ucrit protocol (18), as detailed by Teulier et al. (34).
Lactate kinetics.
Lactate kinetics were measured for 4 or 5 h at rest or during graded swimming (Ucrit test). The rates of lactate appearance (total Ra = endogenous Ra + exogenous Ra) and lactate disposal (Rd) were measured by continuous infusion of [U-14C] lactate (New England Nuclear, Boston, MA; 4.84 GBq/mmol), as previously described (28). The infusates containing labeled lactate were freshly prepared before each experiment and administered at a rate of 1,635 ± 90 Bq·kg−1·min−1 (n = 12) using a calibrated syringe pump (Harvard Apparatus, South Natick, MA) at 1 ml/h. Blood samples (100 μl each) were drawn 50, 55, and 60 min after the start of infusion to ensure that isotopic steady-state had been reached. Additional samples were taken every 15 min (rest) or every 20 min (exercise) until the end of the experiments. The blood sampled from each fish accounted for <10% of total blood volume. Samples were immediately deproteinized in 200 μl of perchloric acid (6% wt/wt) and centrifuged for 5 min at 16,000 g (Eppendorf 5415C, Brinkmann, Rexdale, Canada). Supernatants were stored at −20°C until analyses.
Sample analyses.
Blood lactate and glucose concentrations were measured spectrophotometrically (2) using a Spectra Max plus 384 (Molecular Devices, Sunnyvale, CA). To measure their activities, lactate and glucose were separated, as described previously (28). Before passing through ion-exchange columns, the deproteinized blood samples were neutralized with 1 M potassium bicarbonate. Radioactivity was measured by scintillation counting (Perkin Elmer Tricarb 2910TR, Waltham, MA) in Bio-Safe II scintillation fluid (RPI, Mount Prospect, IL) and was corrected for recovery from the ion-exchange columns.
Calculations and statistics.
Critical swimming speed (Ucrit), total cost of transport (TCOT), and net cost of transport (NCOT) were calculated as previously described (34). TCOT is the total amount of oxygen required to move one unit body mass by one unit distance, and it includes the cost of sustaining life in resting tissues. NCOT is the oxygen cost to power locomotion alone, and it excludes all maintenance costs incurred at rest. The rates of lactate appearance (total Ra) and disposal (Rd) were calculated using the non-steady-state equations of Steele (33). The continuous infusion method used here to quantify metabolite fluxes and its associated calculations have been thoroughly validated for rainbow trout (15, 16) and routinely applied to measure lactate (28, 34), glucose (17, 31, 40), glycerol (3, 22), and lipoprotein fluxes accurately in this species (21). The rate of endogenous lactate appearance was determined by subtracting the rate of exogenous lactate infusion from measured total Ra. Statistical comparisons were performed using one- or two-way repeated-measures analysis of variance (RM-ANOVA) with the Bonferroni post hoc test to determine which means were different from control values. When the assumption of normality or homoscedasticity was not met, the data were normalized by log10 transformation before parametric analysis. Friedman repeated-measures ANOVA on ranks was used with Dunnett's t-test when normality or homoscedasticity were still not met after transformation. All values are presented as means ± SE, and a level of significance of P < 0.05 was used in all tests.
RESULTS
Lactate kinetics in resting fish.
The metabolic rate of resting fish (MO2) remained constant throughout the experiment and averaged 44 μmol O2·kg−1·min−1 (P = 0.67; Fig. 1A). Lactate concentration increased progressively from 1.5 to 13.4 mM (P < 0.05; Fig. 1B), whereas specific activity decreased from 69 to 42 Bq/μmol (P < 0.05; Fig. 1C). Changes in the rates of lactate appearance (total Ra and endogenous Ra) and disappearance (Rd) of resting fish are shown in Fig. 2. Total Ra lactate (endogenous Ra + exogenous Ra) and Rd lactate increased throughout the experiment (P < 0.001; Fig. 2). Endogenous Ra lactate was not affected by exogenous lactate infusion and remained at baseline (P = 0.17; Fig. 2). Initial and final values for MO2, concentrations, and fluxes of resting fish are summarized in Table 1.
Fig. 1.
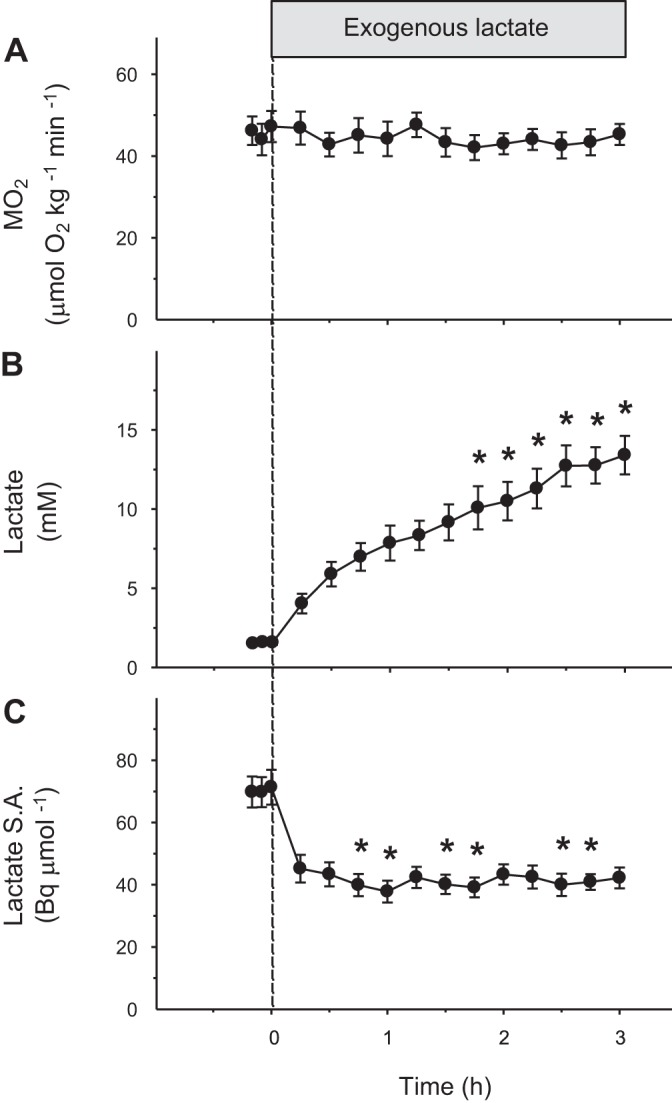
Effects of exogenous lactate infusion on metabolic rate (MO2; A), blood lactate concentration (B), and blood lactate specific activity (lactate S.A.) (C) in resting rainbow trout. These parameters were monitored during the measurement of lactate kinetics by continuous infusion of [U-14C] lactate. Infusion of tracer was started 1 h before time 0 when the infusion of exogenous lactate was initiated. Values are expressed as means ± SE (n = 7). Differences from baseline (time 0) are indicated by *P < 0.05.
Fig. 2.
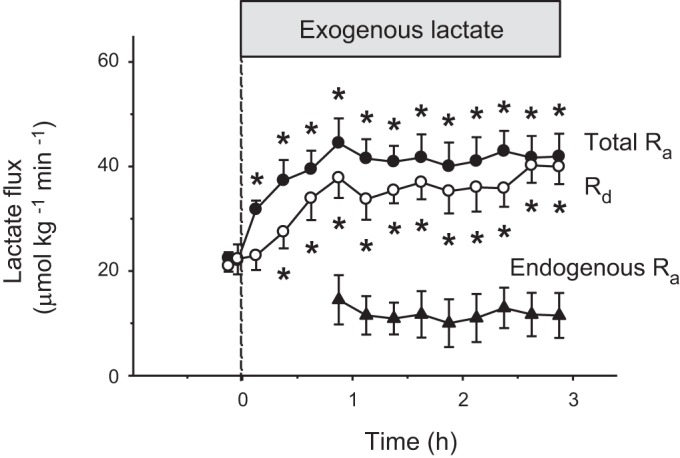
Effects of exogenous lactate infusion on the lactate fluxes of resting rainbow trout. Total rate of appearance (total Ra lactate; ●) is the sum of endogenous lactate production (endogenous Ra; ▲) and exogenous lactate infusion. The rate of lactate disposal (Rd lactate) is shown with open circles (○). Infusion of tracer was started 1 h before time 0 when the infusion of exogenous lactate was initiated. Values are expressed as means ± SE (n = 7). Significant difference from baseline (time 0), *P < 0.001.
Table 1.
Effects of exogenous lactate infusion on blood metabolite concentrations, lactate fluxes, and metabolic rate in rainbow trout at rest and during graded swimming
| Rest |
Graded Swimming |
|||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| Lactate, mM | 1.5 ± 0.1 (7) | 13.4 ± 1.2 (7)* | 1.2 ± 0.2 (5) | 14.7 ± 1.4 (3)* |
| Glucose, mM | 5.2 ± 0.3 (7) | 5.3 ± 0.3 (7) | 4.5 ± 0.4 (5) | 4.6 ± 0.8 (3) |
| Total Ra lactate, μmol·kg−1·min−1 | 22.5 ± 1.1 (7) | 41.9 ± 4.3 (7)* | 21.9 ± 2.7 (5) | 65.3 ± 4.2 (3)*‡ |
| Endogenous Ra lactate, μmol· kg−1·min−1 | 22.5 ± 1.1 (7) | 11.5 ± 4.3 (7) | 21.9 ± 2.7 (5) | 35.3 ± 4.2 (3)*‡ |
| Rd lactate, μmol·kg−1·min−1 | 20.9 ± 1.0 (7) | 39.9 ± 3.3 (7)* | 14.0 ± 1.0 (5) | 56.4 ± 2.0 (3)*‡ |
| MO2, μmol O2 ·kg−1 ·min−1 | 46.2 ± 3.5 (7) | 45.3 ± 2.5 (7) | 52.3 ± 3.1 (5) | 162.0 ± 7.4 (3)*‡ |
Values are expressed as means ± SE with sample sizes in parentheses. MO2, metabolic rate of resting fish; Ra, rate of appearance; total Ra lactate, exogenous lactate supply + endogenous (fish) lactate production; Rd, rate of disposal. Initial values for total Ra and endogenous Ra were identical before exogenous lactate infusion was started. Statistical differences are indicated as
P < 0.05 for initial value vs. final value and
P < 0.05 for resting final value vs. swimming final value.
Metabolic rate, critical swimming speed, and COT of exercising fish.
During graded swimming, the MO2 increased progressively from a baseline value of 47 μmol O2·kg−1·min−1 to a maximum of 257 μmol O2·kg−1·min−1 with increasing speed, but was not different between the controls receiving saline and the treated fish receiving lactate (P = 0.81; Fig. 3A). Exogenous lactate infusion had no effect on Ucrit that was 2.9 ± 0.2 body lengths per second (BL/s) in the control group and 3.0 ± 0.2 BL/s in the lactate group (P = 0.33). TCOT and NCOT were calculated from pooled data from the two treatments (n = 17) because their MO2 was not different. The relationship between TCOT and swimming speed was U-shaped, whereas NCOT increased progressively with speed. Optimal swimming speed (Uopt = speed with minimal COT) was 2.2 BL/s for TCOT and 1.0 BL/s for NCOT. TCOT decreased from a maximal value of 4.2 ± 0.3 μmol O2·kg−1·m−1 to a minimum of 2.6 ± 0.1 μmol O2·kg−1·m−1 (P < 0.01) before returning to 4.0 μmol O2·kg−1·m−1 at the highest speed (Fig. 3B). NCOT increased from 0.9 ± 0.2 μmol O2·kg−1·m−1 to 3.3 μmol O2·kg−1·m−1 at the highest speed (P < 0.01).
Fig. 3.
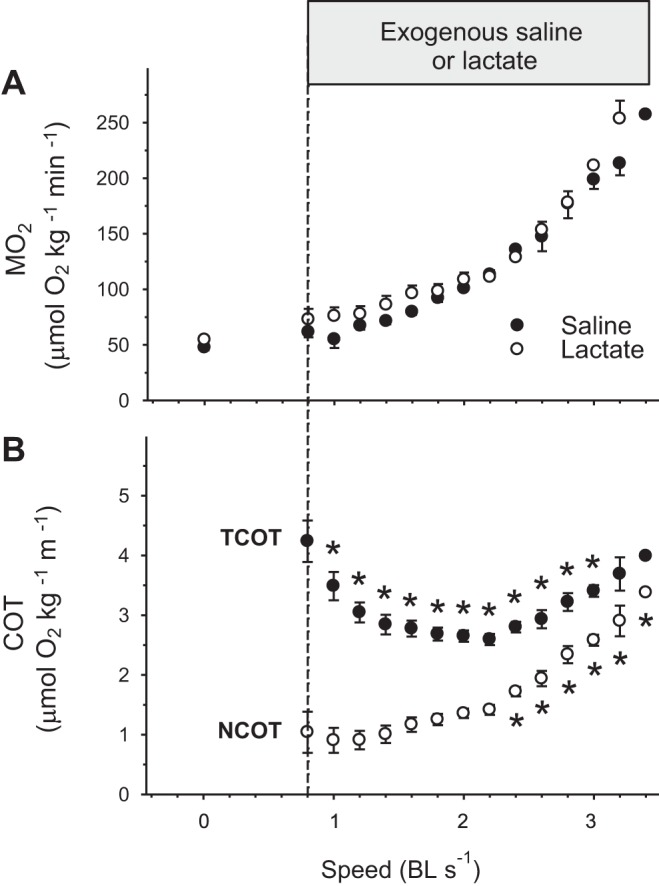
A: MO2 during a critical swimming speed (Ucrit) test for rainbow trout receiving exogenous saline (control; ○) or exogenous lactate (●). Ucrit was not different between the controls [2.9 ± 0.2 body lengths/s (BL/s)] and the lactate group (2.8 ± 0.2 BL/s) (P = 0.33). B: because MO2 was not different between groups (P = 0.81), pooled MO2 values were used to calculate total cost of transport (TCOT; ▲) and net cost of transport (NCOT; △). Values are expressed as means ± SE (n = 8 for lactate and 9 for saline) (n = 17 for TCOT and NCOT). *Significant difference from baseline (0.8 BL/s), P < 0.01.
Lactate kinetics in swimming fish.
With higher speed, MO2 (P < 0.05; Fig. 4A) and blood lactate concentration increased (P < 0.05; Fig. 4B), whereas lactate-specific activity decreased from 117 to 28 Bq/μmol (P < 0.05; Fig. 4C). Changes in total Ra, endogenous Ra, and Rd in animals receiving exogenous lactate during graded swimming are shown in Fig. 5. Total Ra and Rd lactate increased progressively throughout the experiments (P < 0.001). Endogenous Ra lactate only increased at the highest speed (P < 0.05). Initial and final values for these parameters are summarized in Table 1 that also provides a comparison between resting and swimming fish, both receiving exogenous lactate. Total Ra and particularly Rd (that reached 56 μmol·kg−1·min−1) were stimulated much more strongly during exercise than at rest.
Fig. 4.
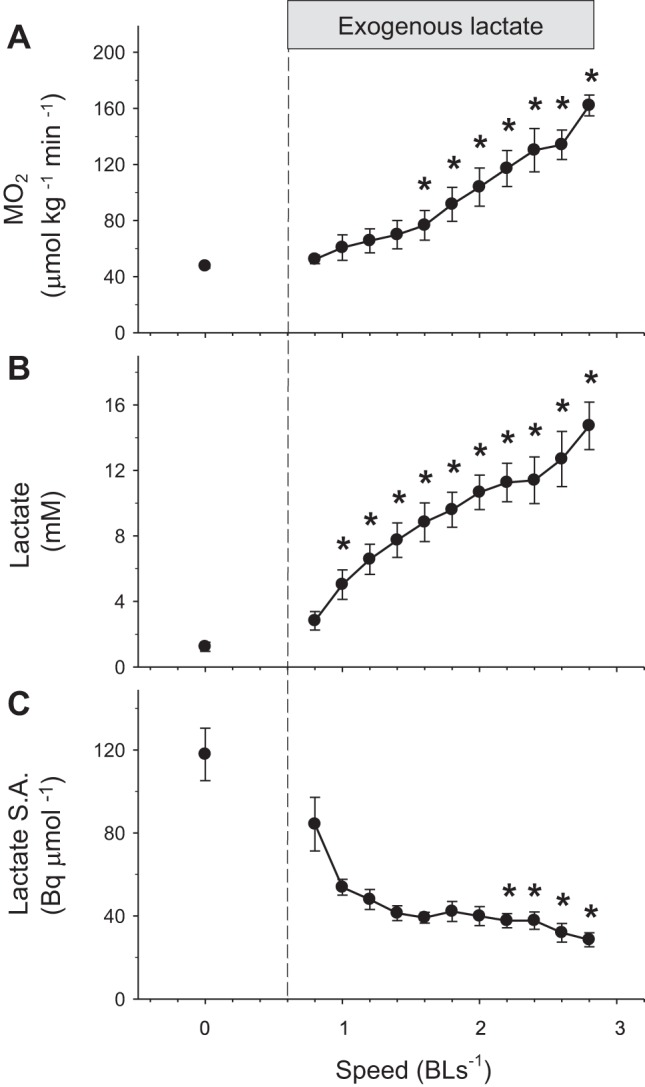
Effects of exogenous lactate infusion on metabolic rate (A), blood lactate concentration (B), and blood lactate-specific activity (lactate S.A.) (C) in rainbow trout during graded exercise. These parameters were monitored during the measurement of lactate kinetics by continuous infusion of [U-14C] lactate. The infusion of exogenous lactate was initiated at the beginning of exercise. Values are expressed as means ± SE (n = 5 or n = 3 for the highest speed that could not be reached by all fish). Significant difference from baseline, *P < 0.05.
Fig. 5.
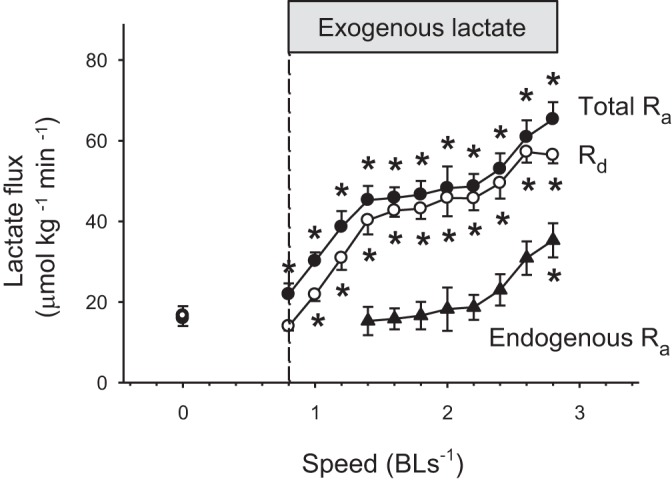
Effects of exogenous lactate infusion on the lactate fluxes of rainbow trout during graded swimming. Total rate of appearance (total Ra lactate; ●) is the sum of endogenous lactate production (endogenous Ra; ▲), and exogenous lactate infusion. The rate of lactate disposal (Rd lactate) is shown with open circles (○). The infusion of exogenous lactate was initiated at the beginning of exercise. Values are expressed as means ± SE (n = 5 or n = 3 for the highest speed). *Significant difference from baseline, P < 0.05.
Glucose metabolism.
Glucose concentration remained constant at baseline (∼5 mM) in control, as well as in swimming fish (P = 0.9; Fig. 6, A and B). The use of lactate as a gluconeogenic precursor was reflected by 14C incorporation into glucose. Glucose-specific activity increased slightly in both groups (P < 0.05; Fig. 6, A and B).
Fig. 6.
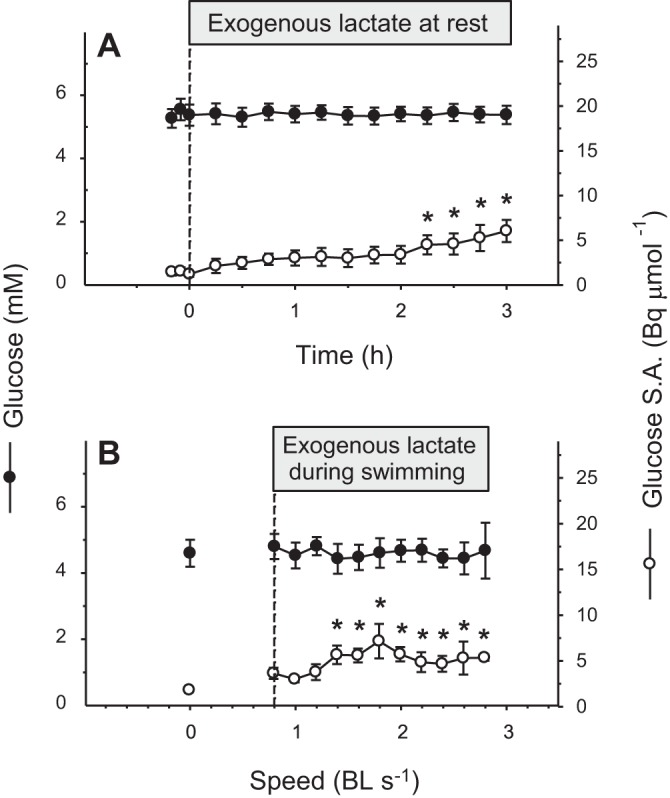
Effects of exogenous lactate infusion on blood glucose concentration (●) and specific activity (○) in rainbow trout at rest (A) or during graded exercise (B). Exogenous lactate infusion was started at time 0 for resting fish (A) and at the beginning of exercise for swimming fish (B). Values are expressed as means ± SE (n = 7 for rest and n = 5 for swimming). *Significant differences from baseline, P < 0.05.
DISCUSSION
This study shows that rainbow trout have a much higher capacity for lactate disposal than previously thought. When cumulating exogenous lactate supply with exercise, they were able to stimulate Rd lactate by 4-fold to reach impressive rates of 56 μmol·kg−1·min−1. Therefore, higher lactate availability allowed them to boost the maximal Rd previously measured after hypoxia or intense swimming by more than 40% (Fig. 7A). Results also reveal that endogenous lactate production is not regulated by circulating lactate concentration, either at rest or during exercise, because Ra lactate is not significantly reduced when exogenous lactate is provided. Increased lactate availability in the circulation does not affect metabolic rate, exercise performance, or swimming energetics, even though this metabolite is a preferred fuel for oxidative tissues (4, 20, 25, 32).
Fig. 7.
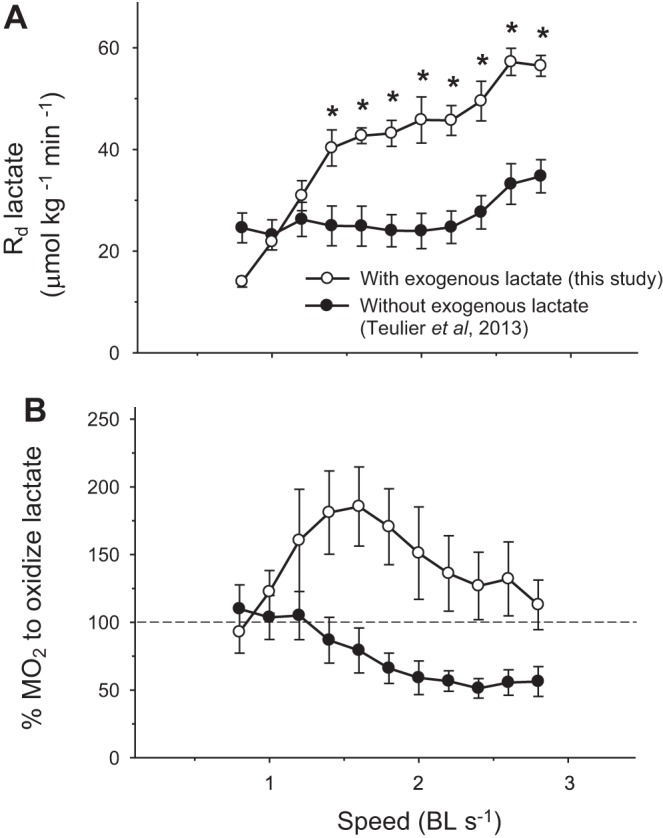
Effects of graded exercise on the rate of lactate disposal (Rd lactate) of rainbow trout. A: fish without exogenous lactate infusion [●; data from (34)] are compared with fish receiving exogenous lactate (○; this study). B: calculated percentage of metabolic rate (%MO2) theoretically needed to oxidize all the lactate leaving the circulation (100% Rd lactate). These percentages show the maximal possible contribution of lactate as an oxidative fuel. Values are expressed as means ± SE (n = 7 for control fish and n = 5 for fish receiving exogenous lactate). *Significant difference from baseline, *P < 0.05.
High capacity for lactate disposal.
In the resting state, providing extra lactate already allowed Rd to reach 40 μmol·kg−1·min−1 (Fig. 2): a higher rate than maximal values measured previously after hypoxia (30 μmol·kg−1·min−1) or intense exercise (34 μmol·kg−1·min−1) (28, 34). More importantly, trout have the reserve capacity to stimulate lactate disposal to 56 μmol·kg−1·min−1 during intense swimming when lactate availability is increased experimentally (Table 1 and Fig. 7A). Such a strong stimulation of Rd is possible because exogenous lactate increases the blood-to-tissue lactate gradient and intracellular lactate concentration in all lactate-utilizing tissues. Here, circulating lactate concentration reached 14 mM, whereas maximal levels after hypoxia or exercise alone were only 5–10 mM. Therefore, transmembrane lactate uptake through the MCTs was stimulated in all tissues that are net users of the end product. We have previously characterized mRNA levels of rainbow trout MCTs (27), showed that they were weakly expressed, and quantified the transcript levels of the dominant isoforms in each tissue [red muscle (MCT1a+b), heart (MCT1a+b and 4), gill (MCT1a), brain (MCT1a and 2), and liver (MCT1a)]. Using a perfused trout trunk preparation, Wang et al. (38) showed that lactate efflux was reduced by alpha-cyano-4-hydroxycinnamate, which was later characterized as an inhibitor of MCTs. Although the Michaelis constant (Km) of trout MCTs has never been measured, such a strong stimulation of Rd suggests that Km values are higher in trout than reported for other species [mammals: 4–7 mM for MCT1 and 0.7 mM for MCT2 (5, 14); fugu, the only fish Km presently known: 4 mM for MCT1b (37)].
Exogenous supply increases lactate availability and, consequently, lactate dehydrogenase activity by mass action effect within the tissues that metabolize the end product during exercise. More pyruvate can then be provided for gluconeogenesis and oxidation: the only two pathways for lactate disposal. The effects of swimming on gluconeogenesis have never been measured in fish, but several studies suggest that it is not stimulated during exercise (reviewed in Ref. 26). This notion is further supported by the fact that hepatic glucose production is partly inhibited during swimming (31) and by the very low incorporation of radioactivity from [U-14C] lactate into glucose (Fig. 6). Stimulating lactate disposal through gluconeogenesis appears undesirable during swimming, because glucose synthesis is energetically costly (6 ATP per glucose) (8). In addition, exogenous lactate infusion has no effect on glycemia, either at rest (Fig. 6A) or during graded exercise (Fig. 6B). At rest, using lactate as a precursor for liver glycogen is also unlikely because a previous study shows that glycogen stores of rainbow trout kept under the same conditions are already quite high (28). Therefore, the strong stimulation of Rd reported here is probably not mediated by gluconeogenesis, but by an increase in lactate oxidation. By contrast, a recent study shows that exogenous lactate stimulates gluconeogenesis in humans, but only during exercise (10). Using in vitro tissue slices (red muscle, liver, heart, kidney, and gills), isolated perfused heart, or brain cells, it was shown that highly aerobic fish tissues readily use lactate as a preferred oxidative fuel (4, 20, 25, 32). Therefore, oxidation is probably responsible for most of the four-fold increase in lactate disposal seen during intense swimming (Table 1 and Fig. 5). Because exogenous lactate has no impact on the metabolic rate of swimming fish (Fig. 3A), it must alter their fuel selection pattern by substituting lactate for other substrates like glucose or fatty acids, although differences in P/O ratios between carbohydrates and lipids might result in a measurable change in MO2. Without a direct measure of lactate oxidation, the role of lactate as an oxidative fuel during swimming cannot be quantified precisely. However, it is possible to calculate the maximal potential contribution of lactate if we assume that 100% of Rd is oxidized. At speeds above 2 BL/s, lactate oxidation could support up to 55% of MO2 in fish receiving no extra lactate, whereas fish that receive lactate could use this fuel exclusively because Rd is more than high enough to account for 100% of MO2 (see Fig. 7B).
Endogenous lactate production and lactate availability.
The high baseline Ra lactate of 20 μmol·kg−1·min−1 measured here is consistent with previous studies on this species (18–24 μmol·kg−1·min−1) (28, 34), but it is unclear why lactate is produced so rapidly under resting conditions in normoxic water. One possible reason could be that lactate must be continually supplied to highly aerobic tissues that favor this fuel for oxidative metabolism (e.g., brain and heart). The numerous lactate shuttles, now well characterized in mammals, emphasize the physiological importance of such a mechanism (7, 12). No information is available on lactate shuttles in fish, and this strikes us as an interesting area for future research. Another reason for maintaining high baseline Ra lactate could be an obligatory need to produce lactate in tissues relying on glycolysis. For example, the gas gland produces lactic acid to release O2 from hemoglobin into the swim bladder by Root effect and control buoyancy (37). In resting fish, the rate of endogenous lactate production is not reduced by exogenous lactate (endogenous Ra in Fig. 2), but a nonsignificant trend toward a decrease is apparent. A nonparametric ANOVA on ranks (with low power) was used for this statistical test because the assumption of normality was not met [if this assumption is ignored, a parametric ANOVA (with higher power) suggests that values after time 1 h could be below baseline (P < 0.05)]. This shows that rainbow trout must continue to release lactate and that some of the baseline production cannot be stopped (e.g., from the gas gland). These results are in agreement with what has been reported for humans by Miller et al. (23) and by Jenssen et al. (19) (no effect of exogenous lactate, but a nonsignificant trend toward a decrease). Another human study by Searle et al. (30) reached the different conclusion that exogenous lactate causes significant inhibition of baseline Ra. However, these results may be unreliable because a bolus injection of radiotracer was used, and this methodology has important shortcomings (34, 42). During graded swimming, endogenous Ra lactate is the same between the fish receiving extra lactate (Fig. 5; 22 to 35 μmol·kg−1·min−1) and those that do not (24 to 40 μmol·kg−1·min−1) (34). This is probably simply because energy metabolism of white muscle must rely equally on anaerobic glycolysis whether circulating lactate availability is high or low.
Exogenous lactate and swimming performance.
Increasing lactate availability has no beneficial or detrimental effect on the exercise capacity of rainbow trout. Fish with or without exogenous lactate reach the same Ucrit (2.8 BL/s) and show the same relationship between metabolic rate and swimming speed (Fig. 3A). Therefore, exogenous lactate does not alter an important index of swimming energetics like TCOT (the total amount of energy required to move one unit body mass by one unit distance). Even NCOT (the energy cost to power locomotion alone, excluding rest) was not modified by exogenous lactate because resting MO2 remained constant (Fig. 1A). Even though lactate is an excellent fuel for oxidative tissues like red muscle (4), trout were unable to improve their swimming performance when lactate was artificially provided at twice the baseline rate of endogenous production. This shows that Ucrit is probably limited by oxygen supply rather than substrate availability. Presumably, the extra lactate did not act as supplementary substrate, but rather as a substitute for other fuels like glucose and lipids. The only other study addressing this issue reached the same conclusion and showed that the performance of human cyclists was not affected (9).
Perspectives and Significance
By using exogenous lactate infusion, we have investigated what constrains maximal lactate disposal and minimal lactate production. Our study is the first to use this approach to examine fuel metabolism in an ectotherm. It shows that metabolic capacity for lactate disposal is not responsible for limiting Rd lactate to the highest levels normally seen after hypoxia or intense swimming because exogenous lactate allows rainbow trout to lift this ceiling by 40% (Fig. 7A). Such a large increase is made possible by accelerating MCT-mediated transport into oxidative tissues and activating their lactate dehydrogenase by mass action effect. The extra pyruvate is then mostly oxidized in trout because hepatic gluconeogenesis is probably not contributing to this response, whereas humans channel a significant fraction of Rd lactate toward glucose synthesis (10). Like mammals, rainbow trout have an obligatory need to produce lactate because baseline endogenous Ra is not significantly reduced by exogenous lactate. It is unclear whether maintaining such a high baseline Ra lactate is associated with an obligatory need for fuel supply to red muscle, heart, and brain or for glycolysis by tissues like the gas gland. Exogenous lactate does not affect aerobic performance (Ucrit) or swimming energetics (COT), probably because it acts as a substitute for glucose or lipids rather than as a supplementary fuel. These observations, put together with the weak expression of MCTs (27) and the classic phenomenon of lactate retention by white muscle after exhausting exercise (35, 38), show that lactate metabolism of rainbow trout is significantly constrained by transmembrane transport. Examining the effects of exogenous lactate on the lactate kinetics of ectothermic animals that do not show muscle lactate retention strikes us as an interesting avenue for future work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.O. and J.-M.W. conception and design of research; T.O. and K.L. performed experiments; T.O. and K.L. analyzed data; T.O., K.L., and J.-M.W. interpreted results of experiments; T.O. prepared figures; T.O. drafted manuscript; T.O. and J.-M.W. edited and revised manuscript; T.O., K.L., and J.-M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Bill Fletcher for taking care of the animals. This work was supported by grants from the Natural Sciences and Engineering Research Council (NSERC) of Canada to J.-M. Weber (NSERC: Discovery, and Research Tools and Instruments grants).
REFERENCES
- 1.Azevedo JL, Tietz E, Two-Feathers T, Paull J, Chapman K. Lactate, fructose and glucose oxidation profiles in sports drinks and the effect on exercise performance. PLoS One 2: 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmeyer HU. Methods of Enzymatic Analysis. Weinheim, Germany: VCH, 1985 [Google Scholar]
- 3.Bernard SF, Reidy SP, Zwingelstein G, Weber JM. Glycerol and fatty acid kinetics in rainbow trout: effects of endurance swimming. J Exp Biol 202: 279–288, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Bilinski E, Jonas REE. Oxidation of lactate to carbon dioxide by rainbow trout (Salmo gairdneri) tissues. J Fish Res Bd Can 29: 1467–1471, 1972 [Google Scholar]
- 5.Bonen A, Tonouchi M, Miskovic D, Heddle C, Heikkila JJ, Halestrap AP. Isoform-specific regulation of the lactate transporters MCT1 and MCT4 by contractile activity. Am J Physiol Endocrinol Metab 279: E1131–E1138, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Brooks GA. Current concepts in lactate exchange. Med Sci Sports Exerc 23: 895–906, 1991 [PubMed] [Google Scholar]
- 7.Brooks GA. Lactate shuttles in nature. Biochem Soc T 30: 258–264, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Clark MG, Bloxham DP, Holland PC, Lardy HA. Estimation of the fructose 1,6-diphosphatase-phosphofructokinase substrate cycle and its relationship to gluconeogenesis in rat liver in vivo. J Biol Chem 249: 279–290, 1974 [PubMed] [Google Scholar]
- 9.Ellis D, Simmons C, Miller BF. Sodium lactate infusion during a cycling time-trial does not increase lactate concentration or decrease performance. Eur J Sport Sci 9: 367–374, 2009 [Google Scholar]
- 10.Emhoff CAW, Messonnier LA, Horning MA, Fattor JA, Carlson TJ, Brooks GA. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J Appl Physiol 114: 297–306, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrannini E, Natali A, Brandi LS, Bonadonna R, De Kreutzemberg SV, DelPrato S, Santoro D. Metabolic and thermogenic effects of lactate infusion in humans. Am J Physiol Endocrinol Metab 265: E504–E512, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Gladden LB. Lactate metabolism: a new paradigm for the third millenium. J Physiol 558: 5–30, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haesler E, Schneiter P, Temler E, Jequier E, Tappy L. Effects of lactate infusion on hepatic gluconeogenesis and glycogenolysis. Clin Physiol 15: 581–595, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Halestrap AP, Meredith D. The SLC16 gene family. From monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflüg Arch 447: 619–628, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Haman F, Powell M, Weber JM. Reliability of continuous tracer infusion for measuring glucose turnover rate in rainbow trout. J Exp Biol 200: 2557–2563, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Haman F, Weber JM. Continuous tracer infusion to measure in vivo metabolite turnover rates in trout. J Exp Biol 199: 1157–1162, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Haman F, Zwingelstein G, Weber JM. Effects of hypoxia and low temperature on substrate fluxes in fish: plasma metabolite concentrations are misleading. Am J Physiol Regul Integr Comp Physiol 273: R2046–R2054, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Jain K, Hamilton J, Farrell A. Use of a ramp velocity test to measure critical swimming speed in rainbow trout (Onchorhynchus mykiss). Comp Biochem Physiol A 117: 441–444, 1997 [Google Scholar]
- 19.Jenssen T, Nurjhan N, Consoli A, Gerich JE. Dose-response effects of lactate infusions on gluconeogenesis from lactate in normal man. Eur J Clin Invest 23: 448–454, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Lanctin HP, McMorran LE, Driedzic WR. Rates of glucose and lactate oxidation by the perfused isolated trout (Salvelinus fontinalis) heart. Can J of Zool 58: 1708–1711, 1980 [DOI] [PubMed] [Google Scholar]
- 21.Magnoni L, Vaillancourt E, Weber JM. High resting triacylglycerol turnover of rainbow trout exceeds the energy requirements of endurance swimming. Am J Physiol Regul Integr Comp Physiol 295: R309–R315, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Magnoni L, Vaillancourt E, Weber JM. In vivo regulation of rainbow trout lipolysis by catecholamines. J Exp Biol 211: 2460–2466, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Miller BF, Fattor JA, Jacobs KA, Horning MA, Navazio F, Lindinger MI, Brooks GA. Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. J Physiol London 544: 963–975, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller BF, Fattor JA, Jacobs KA, Horning MA, Suh SH, Navazio F, Brooks GA. Metabolic and cardiorespiratory responses to “the lactate clamp”. Am J Physiol Endocrinol Metab 283: E889–E898, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Milligan CL, Farrell AP. Lactate utilization by an in situ perfused trout heart: effects of workload and blockers of lactate transport. J Exp Biol 155: 357–373, 1991 [Google Scholar]
- 26.Moyes CD, West TG. Exercise metabolism of fish. Biochem Mol Biol Fishes 4: 367–392, 1995 [Google Scholar]
- 27.Omlin T, Weber JM. Exhausting exercise and tissue-specific expression of monocarboxylate transporters in rainbow trout. Am J Physiol Regul Integr Comp Physiol 304: R1036–R1043, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omlin T, Weber JM. Hypoxia stimulates lactate disposal in rainbow trout. J Exp Biol 213: 3802–3809, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Philp A, Macdonald AL, Watt PW. Lactate—a signal coordinating cell and systemic function. J Exp Biol 208: 4561–4575, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Searle GL, Feingold KR, Hsu FSF, Clark OH, Gertz EW, Stanley WC. Inhibition of endogenous lactate turnover with lactate infusion in humans. Metabolism 38: 1120–1123, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Shanghavi DS, Weber JM. Effects of sustained swimming on hepatic glucose production of rainbow trout. J Exp Biol 202: 2161–2166, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Soengas JL, Aldegunde M. Energy metabolism of fish brain. Comp Biochem Physiol B 131: 271–296, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Steele R. Influence of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82: 420–430, 1959 [DOI] [PubMed] [Google Scholar]
- 34.Teulier L, Omlin T, Weber JM. Lactate kinetics of rainbow trout during graded exercise: do catheters affect the cost of transport? J Exp Biol 216: 4549–4556, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Turner JD, Wood CM. Factors affecting lactate and proton efflux from pre-exercised, isolated-perfused rainbow trout trunks. J Exp Biol 105: 395–401, 1983 [Google Scholar]
- 36.Turner JD, Wood CM, Clark D. Lactate and proton dynamics in the rainbow trout (Salmo gairdneri). J Exp Biol 104: 247–268, 1983 [Google Scholar]
- 37.Umezawa T, Kato A, Ogoshi M, Ookata K, Munakata K, Yamamoto Y, Islam Z, Doi H, Romero MF, Hirose S. O2-filled swimbladder employs monocarboxylate transporters for the generation of O2 by lactate-induced root effect hemoglobin. PLoS One 7: e34579, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Wright PM, Heigenhauser GJF, Wood CM. Lactate transport by rainbow trout white muscle: kinetic characteristics and sensitivity to inhibitors. Am J Physiol Regul Integr Comp Physiol 272: R1577–R1587, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Wardle CS. Non-release of lactic acid from anaerobic swimming muscle of plaice Pleuronectes platessa L.: a stress reaction. J Exp Biol 77: 141–155, 1978 [DOI] [PubMed] [Google Scholar]
- 40.Weber JM, Shanghavi DS. Regulation of glucose production in rainbow trout: Role of epinephrine in vivo and in isolated hepatocytes. Am J Physiol Regul Integr Comp Physiol 278: R956–R963, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Wolf K. Physiological saline for fresh water teleosts. Prog Fish Cult 25: 135–140, 1963 [Google Scholar]
- 42.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine. Principles and Practice of Kinetic Analysis. New York: Wiley Liss, 1992, p. 471 [Google Scholar]


